This paper is shared here for educational purposes and as a reference. Find it here on pubmed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3108032/
The polyvagal theory: New insights into adaptive reactions of the autonomic nervous system
Abstract
The polyvagal theory describes an autonomic nervous system that is influenced by the central nervous system, sensitive to afferent influences, characterized by an adaptive reactivity dependent on the phylogeny of the neural circuits, and interactive with source nuclei in the brainstem regulating the striated muscles of the face and head. The theory is dependent on accumulated knowledge describing the phylogenetic transitions in the vertebrate autonomic nervous system. Its specific focus is on the phylogenetic shift between reptiles and mammals that resulted in specific changes to the vagal pathways regulating the heart. As the source nuclei of the primary vagal efferent pathways regulating the heart shifted from the dorsal motor nucleus of the vagus in reptiles to the nucleus ambiguus in mammals, a face–heart connection evolved with emergent properties of a social engagement system that would enable social interactions to regulate visceral state.
HISTORICAL PERSPECTIVES ON THE AUTONOMIC NERVOUS SYSTEM
Central nervous system regulation of visceral organs is the focus of several historic publications that have shaped the texture of physiological inquiry. For example, in 1872 Darwin acknowledged the dynamic neural relationship between the heart and the brain:
. . .when the heart is affected it reacts on the brain; and the state of the brain again reacts through the pneumo-gastric [vagus] nerve on the heart; so that under any excitement there will be much mutual action and reaction between these, the two most important organs of the body.1
Although Darwin acknowledged the bidirectional communication between the viscera and the brain, subsequent formal description of the autonomic nervous system (eg, by Langley2) minimized the importance of central regulatory structures and afferents. Following Langley, medical and physiological research tended to focus on the peripheral motor nerves of the autonomic nervous sytem, with a conceptual emphasis on the paired antagonism between sympathetic and parasympathetic efferent pathways on the target visceral organs. This focus minimized interest in both afferent pathways and the brainstem areas that regulate specific efferent pathways.
The early conceptualization of the vagus focused on an undifferentiated efferent pathway that was assumed to modulate “tone” concurrently to several target organs. Thus, brainstem areas regulating the supradiaphragmatic (eg, myelinated vagal pathways originating in the nucleus ambiguus and terminating primarily above the diaphragm) were not functionally distinguished from those regulating the subdiaphragmatic (eg, unmyelinated vagal pathways originating in the dorsal motor nucleus of the vagus and terminating primarily below the diaphragm). Without this distinction, research and theory focused on the paired antagonism between the parasympathetic and sympathetic innervation to target organs. The consequence of an emphasis on paired antagonism was an acceptance in physiology and medicine of global constructs such as autonomic balance, sympathetic tone, and vagal tone.
More than 50 years ago, Hess proposed that the autonomic nervous system was not solely vegetative and automatic but was instead an integrated system with both peripheral and central neurons.3 By emphasizing the central mechanisms that mediate the dynamic regulation of peripheral organs, Hess anticipated the need for technologies to continuously monitor peripheral and central neural circuits involved in the regulation of visceral function.
THE VAGAL PARADOX
In 1992, I proposed that an estimate of vagal tone, derived from measuring respiratory sinus arrhythmia, could be used in clinical medicine as an index of stress vulnerability.4 Rather than using the descriptive measures of heart rate variability (ie, beat-to-beat variability) frequently used in obstetrics and pediatrics, the paper emphasized that respiratory sinus arrhythmia has a neural origin and represents the tonic functional outflow from the vagus to the heart (ie, cardiac vagal tone). Thus, it was proposed that respiratory sinus arrhythmia would provide a more sensitive index of health status than a more global measure of beat-to-beat heart rate variability reflecting undetermined neural and nonneural mechanisms. The paper presented a quantitative approach that applied time-series analyses to extract the amplitude of respiratory sinus arrhythmia as a more accurate index of vagal activity. The article provided data demonstrating that healthy full-term infants had respiratory sinus arrhythmia of significantly greater amplitude than did preterm infants. This idea of using heart rate patterns to index vagal activity was not new, having been reported as early as 1910 by Hering.5 Moreover, contemporary studies have reliably reported that vagal blockade via atropine depresses respiratory sinus arrhythmia in mammals.6,7
In response to this article,4 I received a letter from a neonatologist who wrote that, as a medical student, he learned that vagal tone could be lethal. He argued that perhaps too much of a good thing (ie, vagal tone) could be bad. He was referring, of course, to the clinical risk of neurogenic bradycardia. Bradycardia, when observed during delivery, may be an indicator of fetal distress. Similarly, bradycardia and apnea are important indicators of risk for the newborn.
My colleagues and I further investigated this perplexing observation by studying the human fetus during delivery. We observed that fetal bradycardia occurred only when respiratory sinus arrhythmia was depressed (ie, a respiratory rhythm in fetal heart rate is observable even in the absence of the large chest wall movements associated with breathing that occur postpartum).8 This raised the question of how vagal mechanisms could mediate both respiratory sinus arrhythmia and bradycardia, as one is protective and the other is potentially lethal. This inconsistency became the “vagal paradox” and served as the motivation behind the polyvagal theory.
With regard to the mechanisms mediating brady-cardia and heart rate variability, there is an obvious inconsistency between data and physiological assumptions. Physiological models assume vagal regulation of both chronotropic control of the heart (ie, heart rate) and the amplitude of respiratory sinus arrhythmia.9,10For example, it has been reliably reported that vagal cardio-inhibitory fibers to the heart have consistent functional properties characterized by bradycardia to neural stimulation and a respiratory rhythm.9 However, although there are situations in which both measures covary (eg, during exercise and cholinergic blockade), there are other situations in which the measures appear to reflect independent sources of neural control (eg, bradycardic episodes associated with hypoxia, vasovagal syncope, and fetal distress). In contrast to these observable phenomena, researchers continue to argue for a covariation between these two parameters. This inconsistency, based on an assumption of a single central vagal source, is what I have labeled the vagal paradox.
THE POLYVAGAL THEORY: THREE PHYLOGENETIC RESPONSE SYSTEMS
Investigation of the phylogeny of the vertebrate autonomic nervous system provides an answer to the vagal paradox. Research in comparative neuroanatomy and neurophysiology has identified two branches of the vagus, with each branch supporting different adaptive functions and behavioral strategies. The vagal output to the heart from one branch is manifested in respiratory sinus arrhythmia, and the output from the other branch is manifested in bradycardia and possibly the slower rhythms in heart rate variability. Although the slower rhythms have been assumed to have a sympathetic influence, they are blocked by atropine.7
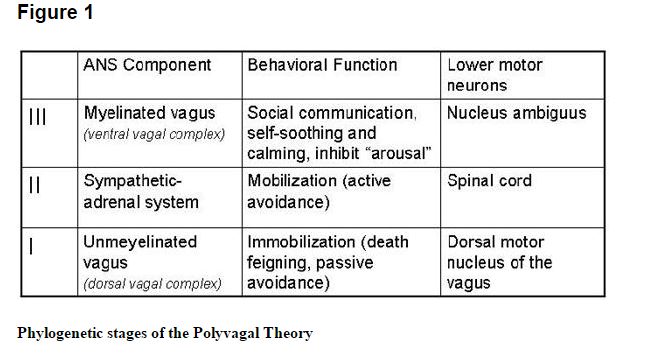
The polyvagal theory7,11–15 articulates how each of three phylogenetic stages in the development of the vertebrate autonomic nervous system is associated with a distinct autonomic subsystem that is retained and expressed in mammals. These autonomic subsystems are phylogenetically ordered and behaviorally linked to social communication (eg, facial expression, vocalization, listening), mobilization (eg, fight–flight behaviors), and immobilization (eg, feigning death, vasovagal syncope, and behavioral shutdown).
The social communication system (ie, social engagement system; see below) involves the myelinated vagus, which serves to foster calm behavioral states by inhibiting sympathetic influences to the heart and dampening the hypothalamic-pituitary-adrenal (HPA) axis.16 The mobilization system is dependent on the functioning of the sympathetic nervous system. The most phylo-genetically primitive component, the immobilization system, is dependent on the unmyelinated vagus, which is shared with most vertebrates. With increased neural complexity resulting from phylogenetic development, the organism’s behavioral and affective repertoire is enriched. The three circuits can be conceptualized as dynamic, providing adaptive responses to safe, dangerous, and life-threatening events and contexts.
Only mammals have a myelinated vagus. Unlike the unmyelinated vagus, originating in the dorsal motor nucleus of the vagus with pre- and postganglionic muscarinic receptors, the mammalian myelinated vagus originates in the nucleus ambiguus and has preganglionic nicotinic receptors and postganglionic muscarinic receptors. The unmyelinated vagus is shared with other vertebrates, including reptiles, amphibians, teleosts, and elasmobranchs.
We are now investigating the possibility of extracting different features of the heart rate pattern to dynamically monitor the two vagal systems. Preliminary studies in our laboratory support this possibility. In these studies we have blocked the nicotinic preganglionic receptors with hexamethonium and the muscarinic receptors with atropine. The data were collected from the prairie vole,17 which has a very high ambient vagal tone. These preliminary data demonstrated that, in several animals, nicotinic blockade selectively removes respiratory sinus arrhythmia without dampening the amplitude of the lower frequencies in heart rate variability. In contrast, blocking the muscarinic receptors with atropine removes both the low and respiratory frequencies.
CONSISTENCY WITH JACKSONIAN DISSOLUTION
The three circuits are organized and respond to challenges in a phylogenetically determined hierarchy consistent with the Jacksonian principle of dissolution. Jackson proposed that in the brain, higher (ie, phylogenetically newer) neural circuits inhibit lower (ie, phylogenetically older) neural circuits and “when the higher are suddenly rendered functionless, the lower rise in activity.”18 Although Jackson proposed dissolution to explain changes in brain function due to damage and illness, the polyvagal theory proposes a similar phylogenetically ordered hierarchical model to describe the sequence of autonomic response strategies to challenges.
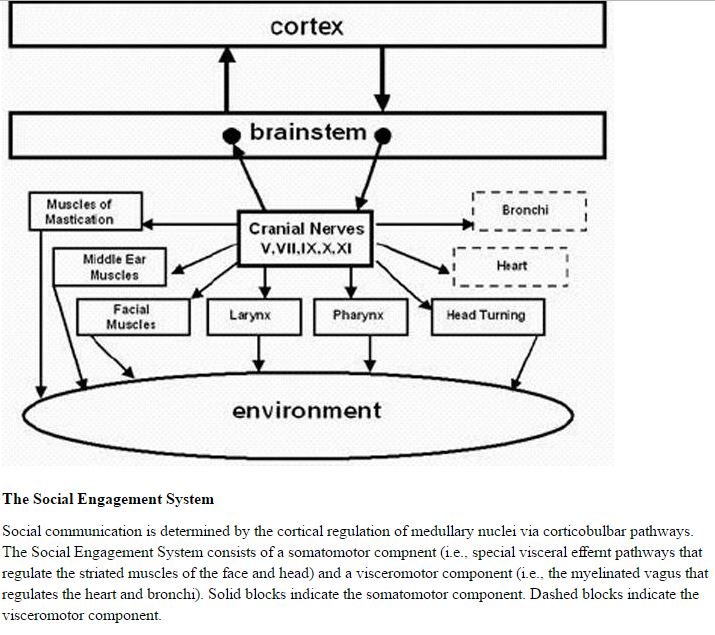
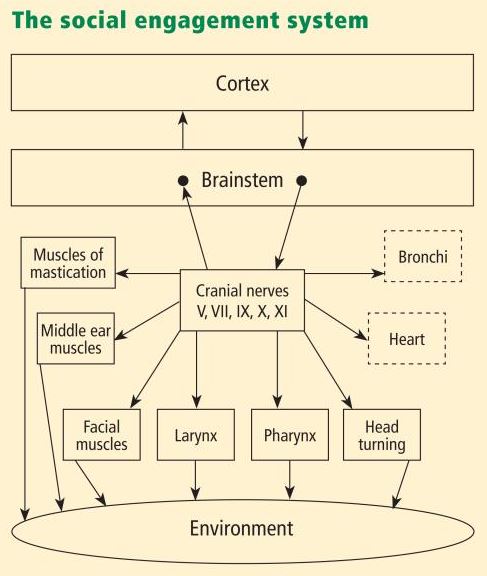
Functionally, when the environment is perceived as safe, two important features are expressed. First, bodily state is regulated in an efficient manner to promote growth and restoration (eg, visceral homeostasis). This is done through an increase in the influence of mammalian myelinated vagal motor pathways on the cardiac pacemaker that slows the heart, inhibits the fight–flight mechanisms of the sympathetic nervous system, dampens the stress response system of the HPA axis (eg, cortisol), and reduces inflammation by modulating immune reactions (eg, cyto kines). Second, through the process of evolution, the brainstem nuclei that regulate the myelinated vagus became integrated with the nuclei that regulate the muscles of the face and head. This link results in the bidirectional coupling between spontaneous social engagement behaviors and bodily states. Specifically, an integrated social engagement system emerged in mammals when the neural regulation of visceral states that promote growth and restoration (via the myelinated vagus) was linked neuroanatomically and neurophysiologically with the neural regulation of the muscles controlling eye gaze, facial expression, listening, and prosody (Figure 1; see Porges7 for review).
The human nervous system, similar to that of other mammals, evolved not solely to survive in safe environments but also to promote survival in dangerous and life-threatening contexts. To accomplish this adaptive flexibility, the human nervous system retained two more primitive neural circuits to regulate defensive strategies (ie, fight–flight and death-feigning behaviors). It is important to note that social behavior, social communication, and visceral homeostasis are incompatible with the neurophysiological states and behaviors promoted by the two neural circuits that support defense strategies. Thus, via evolution, the human nervous system retains three neural circuits, which are in a phylogenetically organized hierarchy. In this hierarchy of adaptive responses, the newest circuit is used first; if that circuit fails to provide safety, the older circuits are recruited sequentially.
Investigation of the phylogeny of regulation of the vertebrate heart11,12,19,20 has led to extraction of four principles that provide a basis for testing of hypotheses relating specifi c neural mechanisms to social engagement, fight–flight, and death-feigning behaviors:
-
There is a phylogenetic shift in the regulation of the heart from endocrine communication to unmyelinated nerves and finally to myelinated nerves.
-
There is a development of opposing neural mechanisms of excitation and inhibition to provide rapid regulation of graded metabolic output.
-
A face–heart connection evolved as source nuclei of vagal pathways shifted ventrally from the older dorsal motor nucleus to the nucleus ambiguus. This resulted in an anatomical and neurophysiological linkage between neural regulation of the heart via the myelinated vagus and the special visceral efferent pathways that regulate the striated muscles of the face and head, forming an integrated social engagement system (Figure 1; for more details, see Porges7,15).
-
With increased cortical development, the cortex exhibits greater control over the brainstem via direct (eg, corticobulbar) and indirect (eg, corticoreticular) neural pathways originating in motor cortex and terminating in the source nuclei of the myelinated motor nerves emerging from the brainstem (eg, specifi c neural pathways embedded within cranial nerves V, VII, IX, X, and XI), controlling visceromotor structures (ie, heart, bronchi) as well as somatomotor structures (muscles of the face and head).
NEUROCEPTION: CONTEXTUAL CUEING OF ADAPTIVE, MALADAPTIVE PHYSIOLOGICAL STATES
To effectively switch from defensive to social engagement strategies, the mammalian nervous system needs to perform two important adaptive tasks: (1) assess risk, and (2) if the environment is perceived as safe, inhibit the more primitive limbic structures that control fight, flight, or freeze behaviors.
Any stimulus that has the potential for increasing an organism’s experience of safety has the potential of recruiting the evolutionarily more advanced neural circuits that support the prosocial behaviors of the social engagement system.
The nervous system, through the processing of sensory information from the environment and from the viscera, continuously evaluates risk. Since the neural evaluation of risk does not require conscious awareness and may involve subcortical limbic structures,21 the term neuroception22 was introduced to emphasize a neural process, distinct from perception, that is capable of distinguishing environmental (and visceral) features that are safe, dangerous, or life-threatening. In safe environments, autonomic state is adaptively regulated to dampen sympathetic activation and to protect the oxygen-dependent central nervous system, especially the cortex, from the metabolically conservative reactions of the dorsal vagal complex. However, how does the nervous system know when the environment is safe, dangerous, or life-threatening, and which neural mechanisms evaluate this risk?
Environmental components of neuroception
Neuroception represents a neural process that enables humans and other mammals to engage in social behaviors by distinguishing safe from dangerous contexts. Neuroception is proposed as a plausible mechanism mediating both the expression and the disruption of positive social behavior, emotion regulation, and visceral homeostasis.7,22 Neuroception might be triggered by feature detectors involving areas of temporal cortex that communicate with the central nucleus of the amygdala and the periaqueductal gray, since limbic reactivity is modulated by temporal cortex responses to the intention of voices, faces, and hand movements. Thus, the neuroception of familiar individuals and individuals with appropriately prosodic voices and warm, expressive faces translates into a social interaction promoting a sense of safety.
In most individuals (ie, those without a psychiatric disorder or neuropathology), the nervous system evaluates risk and matches neurophysiological state with the actual risk of the environment. When the environment is appraised as being safe, the defensive limbic structures are inhibited, enabling social engagement and calm visceral states to emerge. In contrast, some individuals experience a mismatch and the nervous system appraises the environment as being dangerous even when it is safe. This mismatch results in physiological states that support fight, flight, or freeze behaviors, but not social engagement behaviors. According to the theory, social communication can be expressed efficiently through the social engagement system only when these defensive circuits are inhibited.
Other contributors to neuroception
The features of risk in the environment do not solely drive neuroception. Afferent feedback from the viscera provides a major mediator of the accessibility of prosocial circuits associated with social engagement behaviors. For example, the polyvagal theory predicts that states of mobilization would compromise our ability to detect positive social cues. Functionally, visceral states color our perception of objects and others. Thus, the same features of one person engaging another may result in a range of outcomes, depending on the physiological state of the target individual. If the person being engaged is in a state in which the social engagement system is easily accessible, the reciprocal prosocial interactions are likely to occur. However, if the individual is in a state of mobilization, the same engaging response might be responded to with the asocial features of withdrawal or aggression. In such a state, it might be very difficult to dampen the mobilization circuit and enable the social engagement system to come back on line.
The insula may be involved in the mediation of neuroception, since it has been proposed as a brain structure involved in conveying the diffuse feedback from the viscera into cognitive awareness. Functional imaging experiments have demonstrated that the insula plays an important role in the experience of pain and the experience of several emotions, including anger, fear, disgust, happiness, and sadness. Critchley proposes that internal body states are represented in the insula and contribute to states of subjective feeling, and he has demonstrated that activity in the insula correlates with interoceptive accuracy.23
SUMMARY
The polyvagal theory proposes that the evolution of the mammalian autonomic nervous system provides the neurophysiological substrates for adaptive behavioral strategies. It further proposes that physiological state limits the range of behavior and psychological experience. The theory links the evolution of the autonomic nervous system to affective experience, emotional expression, facial gestures, vocal communication, and contingent social behavior. In this way, the theory provides a plausible explanation for the reported covariation between atypical autonomic regulation (eg, reduced vagal and increased sympathetic influences to the heart) and psychiatric and behavioral disorders that involve difficulties in regulating appropriate social, emotional, and communication behaviors.
The polyvagal theory provides several insights into the adaptive nature of physiological state. First, the theory emphasizes that physiological states support different classes of behavior. For example, a physiological state characterized by a vagal withdrawal would support the mobilization behaviors of fight and flight. In contrast, a physiological state characterized by increased vagal influence on the heart (via myelinated vagal pathways originating in the nucleus ambiguus) would support spontaneous social engagement behaviors. Second, the theory emphasizes the formation of an integrated social engagement system through functional and structural links between neural control of the striated muscles of the face and the smooth muscles of the viscera. Third, the polyvagal theory proposes a mechanism—neuroception—to trigger or to inhibit defense strategies.
Acknowledgments
Dr. Porges reported that he has no financial interests or relationships that pose a potential conflict of interest with this article. The preparation of this manuscript was supported, in part, by a grant from the National Institutes of Health (HD 053570).
REFERENCES
Love: an emergent property of the mammalian autonomic nervous system.
Abstract
The evolution of the autonomic nervous system provides an organizing principle to interpret the adaptive significance of mammalian affective processes including courting, sexual arousal, copulation, and the establishment of enduring social bonds. According to the Polyvagal Theory (Porges, 1995, 1996, 1997), the well-documented phylogenetic shift in the neural regulation of the autonomic nervous system passes through three stages, each with an associated behavioral strategy. The first stage is characterized by a primitive unmyelinated visceral vagus that fosters digestion and responds to threat by depressing metabolic activity. Behaviorally, the first stage is associated with immobilization behaviors. The second stage is characterized by the sympathetic nervous system that is capable of increasing metabolic output and inhibiting the visceral vagus to foster mobilization behaviors necessary for ‘fight or flight’. The third stage, unique to mammals, is characterized by a myelinated vagus that can rapidly regulate cardiac output to foster engagement and disengagement with the environment. The mammalian vagus is neuroanatomically linked to the cranial nerves that regulate social engagement via facial expression and vocalization. The Polyvagal Theory provides neurobiological explanations for two dimensions of intimacy: courting and the establishment of enduring pair-bonds. Courting is dependent upon the social engagement strategies associated with the mammalian vagus. The establishment of enduring pair-bonds is dependent upon a co-opting of the visceral vagus from an immobilization system associated with fear and avoidance to an immobilization system associated with safety and trust. The theory proposes that the phylogenetic development of the mammalian vagus is paralleled by a specialized communication, via oxytocin and vasopressin, between the hypothalamus and the medullary source nuclei of the viscera vagus, which facilitates sexual arousal, copulation, and the development of enduring pair-bonds.
A subcortical pathway to the right amygdala mediating “unseen” fear.
Abstract
Neuroimaging studies have shown differential amygdala responses to masked (“unseen”) emotional stimuli. How visual signals related to such unseen stimuli access the amygdala is unknown. A possible pathway, involving the superior colliculus and pulvinar, is suggested by observations of patients with striate cortex lesions who show preserved abilities to localize and discriminate visual stimuli that are not consciously perceived (“blindsight”). We used measures of right amygdala neural activity acquired from volunteer subjects viewing masked fear-conditioned faces to determine whether a colliculo-pulvinar pathway was engaged during processing of these unseen target stimuli. Increased connectivity between right amygdala, pulvinar, and superior colliculus was evident when fear-conditioned faces were unseen rather than seen. Right amygdala connectivity with fusiform and orbitofrontal cortices decreased in the same condition. By contrast, the left amygdala, whose activity did not discriminate seen and unseen fear-conditioned targets, showed no masking-dependent changes in connectivity with superior colliculus or pulvinar. These results suggest that a subcortical pathway to the right amygdala, via midbrain and thalamus, provides a route for processing behaviorally relevant unseen visual events in parallel to a cortical route necessary for conscious identification.
Effects of vagus nerve stimulation on extinction of conditioned fear and post-traumatic stress disorder symptoms in rats
Abstract
Exposure-based therapies help patients with post-traumatic stress disorder (PTSD) to extinguish conditioned fear of trauma reminders. However, controlled laboratory studies indicate that PTSD patients do not extinguish conditioned fear as well as healthy controls, and exposure therapy has high failure and dropout rates. The present study examined whether vagus nerve stimulation (VNS) augments extinction of conditioned fear and attenuates PTSD-like symptoms in an animal model of PTSD. To model PTSD, rats were subjected to a single prolonged stress (SPS) protocol, which consisted of restraint, forced swim, loss of consciousness, and 1 week of social isolation. Like PTSD patients, rats subjected to SPS show impaired extinction of conditioned fear. The SPS procedure was followed, 1 week later, by auditory fear conditioning (AFC) and extinction. VNS or sham stimulation was administered during half of the extinction days, and was paired with presentations of the conditioned stimulus. One week after completion of extinction training, rats were given a battery of behavioral tests to assess anxiety, arousal and avoidance. Results indicated that rats given SPS 1 week prior to AFC (PTSD model) failed to extinguish the freezing response after eleven consecutive days of extinction. Administration of VNS reversed the extinction impairment and attenuated reinstatement of the conditioned fear response. Delivery of VNS during extinction also eliminated the PTSD-like symptoms, such as anxiety, hyperarousal and social avoidance for more than 1 week after VNS treatment. These results provide evidence that extinction paired with VNS treatment can lead to remission of fear and improvements in PTSD-like symptoms. Taken together, these findings suggest that VNS may be an effective adjunct to exposure therapy for the treatment of PTSD.
Introduction
Post-traumatic stress disorder (PTSD) affects 22.4 million Americans and can develop following highly stressful experiences, such as combat or sexual assault.1 Although most individuals who have traumatic experiences exhibit transient symptoms of stress, ~30% of these individuals suffer from symptoms for longer than 1 month and meet the criteria for diagnosis of PTSD.1 According to the fifth edition of the Diagnostic and Statistical Manual, an individual may be diagnosed with PTSD after experiencing or witnessing trauma in addition to presenting the following symptoms: re-experiencing the trauma (that is, experiencing emotional or physical distress in response to reminders of the trauma); avoidance of trauma-related stimuli; negative affect, including loss of interest in enjoyable activities; and heightened startle and arousal. Symptoms must persist for more than 1 month and cause significant social or occupational dysfunction.2 The prevalence of PTSD is greater in individuals who have experienced multiple traumatic events, suggesting that earlier stressors predispose individuals to the development of PTSD following a traumatic event later in life.3, 4, 5
Exposure-based therapies are considered the gold standard of treatment for PTSD.6 The goal of exposure-based therapies is to replace conditioned associations of the trauma with new, more appropriate associations. These therapies are based on Pavlov’s observations that learned associations can be modified with extinction training.7 Despite their demonstrated therapeutic efficacy, exposure-based therapies for PTSD have high nonresponse and dropout rates.8, 9, 10 PTSD patients appear to be resistant to exposure-based therapies because of a generalized extinction deficit.11, 12, 13, 14 Further, PTSD patients are impaired in their ability to extinguish conditioned fears that are acquired in controlled laboratory studies.12, 15, 16 Adjuvant treatments that improve the consolidation of extinction learning may improve the effectiveness of exposure-based therapies.
Vagus nerve stimulation (VNS) was approved by the Federal Food and Drug Administration for the prevention of seizures in patients with drug-resistant epilepsy in 1997. Considering that VNS can enhance memory consolidation in rats and humans,17, 18 we hypothesized that administration of VNS during extinction training could enhance consolidation of an extinction memory. We recently reported that VNS enhanced consolidation of fear extinction following auditory fear conditioning (AFC) and promoted synaptic plasticity in the brain circuitry underlying extinction memory.19, 20 These findings suggest that VNS might also be effective in enhancing extinction memory in a rat model of PTSD. To investigate this possibility, we used the single prolonged stress (SPS) procedure in rats, which models successive traumatic events and increases susceptibility for PTSD-like symptoms following fear conditioning.21 Like PTSD patients, rats subjected to SPS and fear conditioning exhibit impaired extinction of conditioned fear;21, 22 this impairment is specifically seen during consolidation of extinction.23 Rats subjected to SPS show behaviors that can be compared to PTSD symptoms, including re-experiencing the trauma, elevated anxiety, arousal and avoidance.21, 22, 23, 24 Here we investigated the effects of pairing VNS with exposure to the conditioned stimulus (CS) during extinction on the conditioned fear response and on other PTSD-like symptoms in rats subjected to SPS. The findings suggest that VNS enhances extinction and attenuates reinstatement of fear. Furthermore, VNS administration during extinction is associated with a reduction in PTSD-like symptoms 1 week later.
Materials and methods
Animals
All procedures were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals, and were approved by the Institutional Animal Care and Use Committee of the University of Texas at Dallas. Male Sprague-Dawley rats (Taconic, Hudson, NY, USA) weighing 225–250 g on arrival were housed on a 12-h light/dark cycle (lights on at 0700 hours) with access to food and water ad libitum. Only male rats were used, as previous results indicate that female rats are not susceptible to the extinction impairment produced by SPS.24 Criteria for exclusion of rats from the analysis was performance ⩾2 s.d. away from the mean on any task.
To investigate the effects of VNS on extinction and reinstatement of conditioned fear, 22 rats were subjected to the SPS procedure (see ‘Rat model of PTSD’ section below),21 followed 1 week later by AFC. Rats subjected to the SPS procedure and AFC were referred to as ‘PTSD model’ rats. Extinction training began 24 h after AFC. The full course of extinction consisted of 11 days of exposure to the CS without reinforcement. During extinction, odd numbered days (extinction days 1, 3, 5, 7, 9 and 11) were used as tests of conditioned fear; four presentations of the CS were administered and conditioned fear to the CS was measured. On even numbered days (extinction days 2, 4, 6, 8 and 10), VNS or sham stimulation was administered and temporally paired with the four CS presentations. Fourteen of the PTSD model rats were given VNS during extinction, and eight were given sham stimulation (Figure 1a).

Twenty-four rats were given the same AFC and extinction without the SPS procedure. These rats were referred to as ‘AFC alone’ rats. Of these, 14 were given VNS during extinction, and 8 were given sham stimulation during extinction (Figure 1b). Twenty-four hours after extinction day 11, rats were given a single reminder footshock. Reinstatement was tested the following day by measuring freezing in response to the conditioned cue.
To test the effects of VNS on PTSD-like symptoms, separate rats were given AFC with SPS (PTSD model rats, n=16) or without SPS (AFC alone rats, n=16) and exposed to the same 11 days of extinction. Eight rats from each group were given VNS during extinction and eight were given sham stimulation. Seven to ten days after completion of all 11 extinction days, rats were tested on a battery of behavioral tests to examine generalized anxiety, arousal, avoidance and social interaction (Figure 1). The order of test administration was counterbalanced to control for potential interactions. These rats were not given a reminder shock or reinstatement test.
Cuff electrode preparation
Cuff electrodes were prepared as previously described.25 In brief, platinum–iridium wire electrodes were affixed to biocompatible micro-renathane cuffs (1.25 mm inner diameter, 2.5 mm outer diameter, 4.0 mm long). Omnetics four-pin connectors were used to connect the VNS cuff to an AM systems stimulator. Two of the connector pins made contact with the platinum–iridium wires in the cuff in order to deliver stimulation to the vagus nerve.
Surgical implantation of cuff electrode
Surgery protocols are described in detail elsewhere.19, 20, 25 In brief, rats were anesthetized with isoflurane (2% at an oxygen flow rate of 600–800 ml/min). The left vagus nerve was located at the cervical level and isolated from other tissue. The left vagus nerve was selected to avoid stimulation effects on the sinoatrial node. Central activation from the left vagus nerve is bilateral.26 The cuff was placed around the nerve and secured in place with a suture. The platinum–iridium wires were tunneled subcutaneously behind the ear to the top of the head and connected to the omnetics connector, which was affixed to the skull using acrylic, to make the headcap. Cessation of breathing was used to test for correct implantation and effectiveness of the VNS cuff; following implantation, while under anesthesia, current (0.8 mA, 1 s) was applied through the cuff to test for cessation of breathing. If cessation of breathing was not observed, the cuff was adjusted or replaced. For sham rats, surgery was conducted in the same manner to isolate the vagus nerve, but the rats were not implanted with a cuff. Animals were given 1 week to recover following surgery.
Rat model of PTSD
Procedures for SPS were adapted from methods developed by Liberzon et al.21 In brief, rats were restrained for 2 h in a plastic cone. Immediately after restraint, rats were forced to swim in a tank of water (22.0-inch diameter, 20 °C) for 20 min. Following a 15-min recuperation period, rats were placed in a desiccator and exposed to diethyl ether vapor (Sigma, St. Louis, MO, USA) until they became anesthetized and unresponsive. They were immediately returned to their home cages and left socially isolated for 1 week.
AFC
On the first day of AFC, rats were exposed to four pretones (four 9 kHz tones, lasting 30 s, at an intensity of 70 dB, administered without any reinforcement) to assess baseline freezing to the tone. Immediately after the pretones, rats received eight tones coupled with a footshock (1 s, 0.4 mA). The tones were presented at a random inter-stimulus interval of between 120 and 240 s. Each shock was administered at a randomized time during the last 25 s of the 30 s tone presentation. Twenty-four hours later, rats underwent a second day of AFC consisting of eight more tone-shock pairings administered in the same way as the previous day, excluding the pretones. All AFC took place in context A (electric grid floor, no olfactory cue). To compare acquisition of conditioned fear between AFC alone rats and PTSD model rats, sessions were recorded and scored by two researchers blind to the treatment conditions. Freezing during the tone, defined as the cessation of movement aside from breathing,27 was used as a measure of conditioned fear. We chose this AFC protocol to compare findings in the PTSD model to what we have observed in normal rats using a similar protocol. In this previous study, we found that eight tone-shock pairings per day for 2 days, with unpredictable shock timing during the 30 s tone produced conditioned fear that was not fully extinguished after 11 days of extinction in sham-treated controls.19
Extinction days
Twenty-four hours after both days of AFC, rats underwent 11 days of extinction in context B. Context B consisted of the same conditioning chamber, but contained a distinct plexiglas insert to change the texture of the floor and the addition of an odor (peppermint oil). Each day of extinction consisted of four presentations of the CS (tone) in the absence of any reinforcement (shock). Based on evidence that VNS can enhance memory consolidation, extinction was carried out over several days to allow for consolidation of the extinction memory. This extended protocol for extinction more closely resembles a clinical timeline for individuals with PTSD who would undergo multiple days of exposure-based therapies. Two observers who were blind to the treatment groups measured the percent of time spent freezing during each 30-s tone, which was recorded as the measure of conditioned fear. Freezing times below 10% of the total 120 s of tone exposure was considered remission of fear.19 During extinction, rats initially respond to being connected to the stimulator, this occurs in both sham-treated and VNS-treated rats. This response is typically present only during the first extinction session. To avoid potential performance effects of VNS or sham stimulation, conditioned fear responses were measured only on alternate, non-stimulation days (extinction days 1, 3, 5, 7, 9 and 11) when the rats were not connected to the stimulator (Figure 1). Although we have not systematically measured unintended effects of VNS, we have noticed that both sham- and VNS-treated rats occasionally attend to the connector after it is attached to the headcap. This variable behavior could interfere with the conditioned response. In addition, measuring conditioned fear on days when rats were not receiving VNS or sham stimulation made it less likely that interoceptive state-dependent effects of VNS could serve as a safety signal, and provided an opportunity to observe the effect of VNS on consolidation of extinction memory.
VNS and sham stimulation
Treatment with VNS or sham stimulation was given during extinction on even numbered days (extinction days 2, 4, 6, 8 and 10). To administer stimulation, an AM systems stimulator was connected to the cuff connector on the headcap via a 25.0 cm long PVS multiconductor cable (Cooner Wire, Los Angeles, CA, USA). Stimulation started 150.0 ms before the onset of each tone and then continued for the duration of the tone. VNS was given at a frequency of 20Hz, an intensity of 0.4 mA for 30 s with a 100 μs pulse width. Sham-treated rats were connected to the stimulator in the same way as VNS-treated rats, but did not receive stimulation.
Reinstatement
Following the completion of extinction, reinstatement of conditioned fear was tested. Twenty-four hours after the 11th day of extinction, rats (N=44) were placed in context A and given one unsignaled footshock (unconditioned stimulus) delivered for 1 s at 0.4 mA intensity, in the absence of the tone (CS). Rats remained in context A for 5 min after the footshock. To observe the reaction to the reinstatement shock, sessions were recorded and scored by two researchers blind to the treatment conditions. Freezing was recorded during the entire 5-min observation period. Twenty-four hours after administration of the unconditioned stimulus, rats were exposed to the CS in context B, to test for reinstatement of fear.
Elevated plus maze
To test generalized anxiety, rats were placed on the central part of an elevated plus-shaped maze (10.0 cm wide, 50.0 cm long, 55.0 cm off the floor) with walls (30.0 cm tall) on two opposing arms and no walls on the other opposing arms. During a 10-min test, time spent in the open arms, time spent in the closed arms and time spent in the center of the maze were recorded. Rats were considered to be in an arm when all four paws were in that arm at one time. All behavior was recorded and scored by two blind researchers. Time spent in the open arms and entries into the open arms were taken as a measure of risk taking.28 Percent of total time spent moving was taken as a control measure of general locomotion.
Acoustic startle response
Before startle behaviors were measured, rats were placed into the apparatus for 5 min to habituate them to the cage. On the following day, rats were placed into the same 20 × 20 × 20 cm3 wire-mesh cage centered on a startle platform (Lafayette Instrument, Lafayette, IN, USA) that used a piezoelectric transducer to generate a continuous record of the rat’s activity. Startle responses were elicited by 50.0 ms bursts of white noise at 95 dB sound pressure level. Each rat was subjected to 20 presentations of the startle stimulus with an inter-stimulus interval of 180 s. The waveform of each response served as the measure of the startle response.
Marble burying
To test novel object avoidance, rats underwent a marble burying task. Rats exposed to a noxious object in their homecage will vigorously bury that object, a phenomenon known as defensive burying.29 This avoidance behavior can be seen in rats following fear conditioning with non-salient, novel objects.30 This defensive burying of novel objects is sensitive to anxiolytic treatments and is used to measure anxiety and avoidance behavior.31 Following habituation to the novel bedding, rats were individually placed into a cage that was identical to their homecage with BioFresh nitrocellulose comfort bedding (3.0 cm deep). Fifteen identical, shiny marbles were placed in three rows in the rear third of the cage. After 10 min, the number of marbles buried was counted and recorded as a measure of novel stimulus avoidance. Only marbles that were more than 2/3 covered by bedding were considered buried. Percent of marbles buried = (number marbles buried/number of marbles present) × 100.
Social interaction
A three-chamber social interaction task was used to assess social behaviors. The apparatus consisted of three equal-sized chambers: the nonsocial zone, the social zone and the center. The nonsocial zone contained a small wire cage that was empty and sealed; the social zone contained an identical wire cage with a stimulus rat inside. The stimulus rat was matched in size and sex to the experimental rat. The experimental rat was placed into the center of the apparatus, facing the nonsocial zone, and allowed to explore for 10 min. Interactions of the experimental rat with the stimulus rat, time spent in the nonsocial zone, time spent in the social zone and time spent in the center were recorded. A rat was considered to be in a zone of the apparatus only when all four paws were in that zone at once. All behavior was recorded and scored by two experimenters who were blind to treatment conditions. The sociability index (time spent in the social zone – time spent in the nonsocial zone)/(time spent in the social zone + time spent in the nonsocial zone) was used to indicate a preference to interact with or avoid the stimulus rat.
Data analysis
Data were analyzed using a two-way repeated measures ANOVA or a one-way ANOVA, with a Greenhouse-Geisser correction followed by a Tukey’s post hoc test for multiple comparisons or a Holm–Bonferroni sequential correction test for non-independent samples. Statistically significant effects were defined as those with P-values that were <0.05. All error bars represent standard error of the mean.
Results
VNS administration during exposure to the conditioned stimulus enhanced extinction and reduced reinstatement of conditioned fear
We modeled PTSD by combining SPS with AFC 1 week later. A two-factor repeated measures ANOVA indicated a significant effect of group across days (F(18 246)=4.764, P<0.0001). Although animals with and without SPS exposure demonstrated comparable levels of conditioned fear following AFC, SPS treatment resulted in significantly higher levels of freezing in response to the CS after 11 consecutive days of extinction (Figure 2a, sham). Without VNS, the PTSD model rats did not reach remission of conditioned fear (<10% freezing to the CS). Administration of VNS treatment during five out of the eleven extinction days led to remission of CS-evoked freezing behavior in PTSD model rats (Figure 2a, VNS). By extinction day 5, PTSD model rats given VNS showed decreased freezing versus rats given sham stimulation (P<0.01). This effect continued until the completion of treatment: extinction day 7 (P<0.0001); extinction day 9 (P<0.0001); and extinction day 11 (P<0.0001). This supports the hypothesis that VNS treatment can enhance extinction of conditioned fear in an animal model of PTSD.

A single reminder of the unconditioned stimulus (reinstatement footshock on day 12) was sufficient to increase freezing to the CS when presented 24 h later. In PTSD model rats given sham stimulation, the level of conditioned fear returned to that observed before any extinction; freezing behavior on extinction day 13 was not significantly different from extinction day 1 (P>0.05). This result indicates that a single stressor is sufficient to restore strong fear behavior in PTSD model rats despite a long period of extinction. The addition of VNS during extinction prevented the reinstatement of conditioned fear observed in PTSD model rats. Freezing behavior in PTSD model rats given VNS was dramatically reduced on extinction day 13 compared to extinction day 1 (P<0.00001) (Figure 2a). This result indicates that VNS during extinction makes PTSD model rats resilient to stress-induced relapse.
To compare the fear demonstrated by the PTSD model rats, we examined fear in rats that underwent AFC in the absence of SPS (AFC alone rats). These rats exhibited remission of fear behavior (⩽10% freezing) and resistance to reinstatement (Figure 2b, sham); freezing behavior in sham-treated rats was dramatically reduced on extinction day 13 compared to extinction day 1 (P<0.00001). These results indicate that the stability and degree of fear extinction is substantially different between PTSD model rats and AFC alone rats, as previously reported.21, 22, 23
Still, in rats that received AFC alone, VNS during extinction accelerated extinction of conditioned fear (Figure 2b). On average, VNS-treated rats reached remission of fear 2 days earlier than sham-treated rats (P<0.01). In rats given AFC alone, VNS treatment reduced freezing versus sham on extinction day 5 (P<0.001) and extinction day 7 (P<0.05). Freezing following reinstatement was not different between the sham- and VNS-treated rats (P>0.05).
Prior to VNS- or sham-paired extinction, rates of acquisition of conditioned fear between AFC alone rats and PTSD model rats were examined (Figure 3a). A two-factor repeated measures ANOVA indicated a significant effect of group across tones for acquisition of conditioned fear (F(7, 168)= 2.473, P<0.05). All rats show a significant effect of time, as levels of freezing increase as the number of tone-shock pairings increases (F(7, 168)=41.36, P<0.0001). A Holm–Bonferroni sequential correction revealed a significant difference between PTSD model rats and AFC alone rats only at tones 9 and 10 (P<0.01). PTSD model rats showed a deficit in fear retention versus AFC alone rats (P<0.01) at the start of the second day of fear conditioning. This could be explained by evidence that SPS impairs consolidation, but has no learning effect within a session.23 This effect was not present during other AFC tones or at the start of extinction training (extinction day 1).

A one-way ANOVA revealed no significant effect of freezing following the reinstatement shock (F(2.582, 8.94)=0.522, P>0.05). All rats showed similar levels of conditioned fear immediately following reinstatement shock (Figure 3b), indicating the shock was equally aversive to all rats. However, 24 h later, there was a significant difference between PTSD model rats given sham stimulation and all other rats (P<0.0001).
A total of two AFC alone rats (1 sham and 1 VNS) met the exclusion criteria. These rats were not included in analysis because they did not exhibit conditioned fear following AFC (freezing behavior was <2 s.d. away from the mean).
VNS administration during extinction sessions reduced PTSD-like symptoms
EPM
To test the effect of VNS on general anxiety, rats were tested on the elevated plus maze (EPM) (Figures 4a and b). A one-way ANOVA revealed a significant effect across groups (F(2.430, 17.01)=17.26, P<0.0001). PTSD model rats given sham stimulation spent less time in the open arms than AFC alone rats given sham stimulation (P<0.05), and made fewer entries into the open arms (P<0.01), indicating that anxiety was elevated in the PTSD model rats. VNS treatment reversed this effect, in that PTSD model rats given VNS during the extinction sessions spent more time in the open arms (P<0.01), and made more entries into the open arms versus PTSD model rats given sham stimulation (P<0.001). PTSD model rats given VNS during extinction sessions spent a similar amount of time in the open arms as AFC alone rats (P>0.05). VNS treatment also increased time spent in the open arms (P<0.05) and entries into the open arms in AFC alone rats (P<0.05). These results demonstrate that VNS treatment during extinction reduced general anxiety 1 week after treatment. Total locomotion was not different in PTSD model rats versus AFC alone rats, and administration of VNS did not affect total locomotion (Figure 4c).
Acoustic startle response
To test for hyperarousal, rats underwent an acoustic startle response test. VNS treatment during extinction reduced startle responses in PTSD model rats and in AFC alone rats (Figure 4d). A one-way ANOVA indicated a significant effect across groups (F(1.40, 9.80)=6.980, P<0.05). Startle amplitudes prior to habituation (the first 15 startle bursts) were similar in PTSD model rats given sham stimulation and sham-treated AFC alone rats (P>0.05). PTSD model rats that received VNS during extinction showed a reduction in startle amplitude versus sham-treated rats (P<0.05). VNS also decreased startle amplitude versus sham-treated AFC alone rats (P<0.05). These results demonstrate that although SPS did not increase startle responses, VNS was still effective in reducing startle amplitude.
Marble burying
To test for avoidance of novel objects, rats were tested on a marble burying task. VNS treatment during extinction reduced avoidance in PTSD model rats (Figure 5a). A one-way ANOVA indicated a significant effect across groups F(2.694, 18.86)=6.622, P<0.01. PTSD model rats given sham stimulation buried more marbles than AFC alone rats (P<0.01). PTSD model rats given VNS during extinction buried fewer marbles than those given sham stimulation (P<0.05), and buried a similar number of marbles as AFC alone rats (P>0.05). This indicates that VNS treatment during extinction reduced novel avoidance behavior in PTSD model rats.

In AFC alone rats, there was no difference between VNS and sham stimulation (P>0.05).
Social interaction
To test whether VNS during extinction could reverse abnormal social interactions characteristic of PTSD, rats were evaluated using a social interaction test. PTSD model rats showed social withdrawal. VNS during extinction reversed the social withdrawal and restored normal social behavior (Figure 5b). A one-way ANOVA indicated a significant effect across groups F(2.632, 18.42)=59.44, P<0.0001. PTSD model rats given sham stimulation had a negative sociability index, indicating the typical preference to interact with the stimulus rat was deficient. The sociability index for the PTSD model rats was significantly lower than that of AFC alone rats (P<0.00001). This was reversed by VNS; PTSD model rats given VNS had a higher sociability index than those given sham stimulation (P<0.0001), and the sociability index was not significantly different from that of AFC alone rats. These results show that VNS treatment during extinction improved social interaction in PTSD model rats. There was no significant difference between social interaction indexes of VNS- versus sham stimulation-treated AFC alone rats.
Taken together, these results demonstrate that anxiety-related behavior in PTSD model rats is qualitatively and quantitatively distinct from that of AFC alone rats.
Discussion
The SPS rat model of PTSD shares important characteristics with PTSD in human subjects. For clinical diagnosis of PTSD, patients must exhibit symptoms from each of the four criteria for more than 1 month.2PTSD patients show extinction impairments that may be responsible for the persistence of fear and anxiety symptoms. Here we observed that exposure to SPS (restraint, swim stress, loss of consciousness and social isolation) 7 days prior to fear conditioning makes rats susceptible to impaired extinction of the fear response. This is consistent with previous observations of extinction impairments in the SPS model.22, 23 However, we found that SPS combined with subsequent AFC lead to a fear response to the CS even after 11 days of extinction. PTSD model rats also showed significantly higher reinstatement, a measure of relapse, following a reminder of the unconditioned stimulus. One of the symptom clusters of PTSD is intrusion symptoms, such as distress and re-experiencing after exposure to traumatic reminders. The present findings indicate that the animal model of PTSD demonstrates resistance to extinction learning and re-experiencing the trauma (inferred from the freezing response) in the presence of reminders of the trauma.
Alterations in arousal and reactivity, such as exaggerated startle responses, make up the second symptom cluster. One week after completion of extinction, PTSD model rats showed heightened anxiety on an EPM, but the acoustic startle response was not significantly different in PTSD model rats and AFC alone rats. PTSD patients demonstrate hypervigilance and exaggerated startle responses. It is possible that auditory fear conditioning alone increases the acoustic startle response, obscuring the effect of multiple stressors. For example, the acoustic startle response is potentiated in fear-conditioned rats and humans when it is tested in the presence of conditioned cues.32, 33 Avoidance is another symptom cluster that is described in the Diagnostic and Statistical Manual-5. PTSD model rats showed an increase in the novel avoidance task of marble burying.
The fourth symptom cluster of PTSD is negative alterations in cognition or mood, such as social withdrawal and persistent negative emotions. Social interaction scores were significantly lower for PTSD model rats than they were for AFC alone rats. In fact, AFC alone rats showed a strong preference for the social zone in the social interaction test, whereas PTSD model rats did not show a preference at all for the social zone over the nonsocial zone. These findings provide evidence of social withdrawal and less engagement in normal activities in the PTSD model. Taken together, these findings suggest that the SPS model of PTSD shows many behaviors that resemble PTSD symptoms, and may be useful in the study of the effects of traumatic events on the brain and behavior.
VNS administration during extinction reversed the extinction impairment observed in PTSD model rats, and VNS improved symptoms from each PTSD symptom cluster, including re-experiencing fear, elevated anxiety, arousal, avoidance and social withdrawal. PTSD model rats continued to exhibit a freezing response to the CS after 11 consecutive days of extinction. Others have shown enhancement in conditioned fear following contextual fear conditioning in the SPS model.34 The extinction impairment in PTSD model rats reported here cannot be explained by a conditioning enhancement, as PTSD model rats do not show an enhancement in conditioning, in fact they show a temporary deficit in fear retention during tones 9 and 10. VNS administration during extinction reversed the extinction impairment in the rat PTSD model. Like AFC alone rats, PTSD model rats given VNS during extinction demonstrated remission of conditioned fear. This reduction in conditioned fear was also observed following reinstatement. A single reminder of the unconditioned stimulus was sufficient to fully reinstate conditioned fear in PTSD model rats, but VNS treatment during extinction prevented this relapse. These findings suggest that VNS may facilitate progress in exposure therapy by enhancing extinction of conditioned fear and reducing relapse.
Although persistent fear in the presence of reminders of the trauma is a well-recognized PTSD symptom, generalized anxiety, hyperarousal and avoidance behaviors can also be disabling. The administration of VNS during extinction reduced anxiety and avoidance behavior 1 week later on tasks that did not involve the CS. The observation that VNS treatment reduced avoidance of novel stimuli and startle responses, and increased exploratory and social behavior in PTSD model rats suggests that this adjuvant therapy can improve pathological behaviors that are not directly related to specific trauma cues.
Extinction is the goal of exposure-based therapies and VNS enhances extinction. However, the mechanisms by which VNS enhances extinction are not yet known. VNS enhances memory consolidation17, 18, 19 and alters the release of neuromodulators into the brain that may promote experience-dependent plasticity.20, 35,36, 37, 38, 39 Pairing VNS with an auditory stimulus alters auditory cortical maps while pairing VNS with motor learning modulates maps in the motor cortex, indicating that the nature of the plasticity is driven by the training that is paired with VNS.40, 41, 42, 43, 44 We recently reported that VNS promotes plasticity in the pathway from the infralimbic area of the prefrontal cortex to the basolateral complex of the amygdala in rats.20, 45 Humans with PTSD exhibit reduced activation of the ventromedial prefrontal cortex and increased activation of the amygdala.46, 47 In addition, extinction impairments, like those observed in rats exposed to SPS, are associated with decreased prefrontal cortical control over amygdala activity.48, 49 VNS enhancement of consolidation of the extinction memory via facilitation of plasticity in this circuitry could be responsible for successful extinction following VNS in PTSD model rats.
VNS may also enhance extinction by inhibiting activity of the sympathetic nervous system.50, 51 The vagus nerve is sometimes referred to as the ‘vagal brake’ as activation of the vagus nerve activates the parasympathetic system and slows heart rate following the sympathetic stress response.52 One study showed that chronic VNS reduced a measure of anxiety in rats,53 and another suggested that chronic VNS improved scores on the Hamilton Anxiety Scale in human patients suffering from treatment-resistant depression.54 It is possible that an immediate VNS-induced reduction in anxiety contributes to VNS-driven extinction by interfering with the sympathetic response to the CS, thus breaking the association of the CS with fear. In addition, a total of 20 trains of VNS administered over the course of 11 days may be sufficient to produce lasting anxiolytic effects, as has been observed following chronic VNS. Such a long-lasting anxiolytic effect would explain the reduction of general PTSD-like symptoms in VNS-treated rats. However, it is not likely that a general and lasting anxiolytic effect is responsible for VNS-driven remission of fear as unpaired administration of VNS did not enhance extinction of conditioned fear in a previously reported study.19
These findings demonstrate that VNS treatment can reverse extinction impairments and provide benefits across a variety of symptoms in a rat model of PTSD. Extinction of conditioned fear in nonhuman animals is frequently used as a preclinical model of exposure therapy.13, 55, 56, 57 The present findings suggest that VNS may be an effective adjunct to exposure therapy. Since VNS has been used in tens of thousands of patients with drug-resistant epilepsy58 and delivery during exposure therapy requires considerably less stimulation, VNS may be safely used to enhance extinction in the treatment of PTSD and other disorders that show improvements with exposure-based therapies.
Acknowledgments
We thank Israel Liberzon, for assistance with the SPS model, and Seth Hays and Steve Maren for valuable discussion and advice. We thank Phillip Gonzales, Dr Rimenez de Souza, Arturo Delgado, Amber Mawji, Ashleigh Chuah and Linda Zhong for all of their technical help and dedication and Drs James McGaugh and Jarid Goodman for reading and providing feedback on a draft of this manuscript. This work was sponsored by the Defense Advanced Research Projects Agency (DARPA) Biological Technologies Office (BTO) Electrical Prescriptions (ElectRx) program under the auspices of Dr Doug Weber through the Space and Naval Warfare Systems Center, Pacific. Grant/Contract No. DARPA-BAA-14-38 and DARPA-BAA-15-06 and by the NIMH, MH105014.
Footnotes
RLR is an owner of Vulintis Inc. and Optokinetics. RLR is a consultant for Konan Medical USA. None of these financial interests are related to this work. MPK is a paid consultant for and shareholder of MicroTransponder. MPK and CKM are authors of a patent entitled ‘Enhancing Fear Extinction using Vagus Nerve Stimulation’. The remaining authors declare no conflict of interest.
References
- Kessler RC, Chiu WT, Demler O, Walters EE. Prevalence, severity, and comorbidity of twelve-month DSM-IV disorders in the National Comorbidity Survey Replication (NCS-R). Arch Gen Psychiatry2005; 62: 617–627. [PMC free article] [PubMed]
- American Psychiatric AssociationDiagnostic and Statistical Manual of Mental Disorders.5th edn, American Psychiatric Publishing: Arlington, VA, USA, 2013.
- Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, Friedman MJ. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J Trauma Stress 2013; 26: 537–547. [PMC free article] [PubMed]
- Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD et al. The enduring effects of abuse and related adverse experiences in childhood. A convergence of evidence from neurobiology and epidemiology. Eur Arch Psychiatry Clin Neurosci 2006; 256: 174–186. [PMC free article][PubMed]
- Maercker A, Michael T, Fehm L, Becker ES, Margraf J. Age of traumatisation as a predictor of post-traumatic stress disorder or major depression in young women. Br J Psychiatry 2004; 184: 482–487.[PubMed]
- Management of Post-Traumatic Stress Working GroupVA/DoD Clinical Practice Guideline for Management of Post-Traumatic Stress. Department of Veterans Affairs, Department of Defense: Washington, DC, 2010.
- Pavlov PI. Conditioned reflexes: an investigation of the physiological activity of the cerebral cortex. Ann Neurosci 2010; 17: 136–141. [PMC free article] [PubMed]
- Kar N. Cognitive behavioral therapy for the treatment of post-traumatic stress disorder: a review. Neuropsychiatr Dis Treat 2011; 7: 1–5. [PMC free article] [PubMed]
- Schottenbauer MA, Glass CR, Arnkoff DB, Tendick V, Gray SH. Nonresponse and dropout rates in outcome studies on PTSD: review and methodological considerations. Psychiatry 2008; 71: 134–168.[PubMed]
- Steenkamp MM, Litz BT, Hoge CW, Marmar CR. Psychotherapy for military-related PTSD: a review of randomized clinical trials. JAMA 2015; 314: 489–500. [PubMed]
- Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biol Psychol 2006; 73: 61–71. [PubMed]
- Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, Pitman RK. Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. J Psychiatr Res 2008; 42: 515–520. [PMC free article] [PubMed]
- Rothbaum BO, Davis M. Applying learning principles to the treatment of post-trauma reactions. Ann N Y Acad Sci 2003; 1008: 112–121. [PubMed]
- Davis M, Myers KM, Chhatwal J, Ressler KJ. Pharmacological treatments that facilitate extinction of fear: relevance to psychotherapy. NeuroRx 2006; 3: 82–96. [PMC free article] [PubMed]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry 2009; 66: 1075–1082. [PMC free article] [PubMed]
- Linnman C, Zeffiro TA, Pitman RK, Milad MR. An fMRI study of unconditioned responses in post-traumatic stress disorder. Biol Mood Anxiety Disord 2011; 1: 8. [PMC free article] [PubMed]
- Clark KB, Krahl SE, Smith DC, Jensen RA. Post-training unilateral vagal stimulation enhances retention performance in the rat. Neurobiol Learn Mem 1995; 63: 213–216. [PubMed]
- Clark KB, Naritoku DK, Smith DC, Browning RA, Jensen RA. Enhances recognition memory following vagus nerve stimulation in human subjects. Nat Neurosci 1999; 2: 94–98. [PubMed]
- Peña DF, Engineer ND, McIntyre CK. Rapid remission of conditioned fear expression with extinction training paired with vagus nerve stimulation. Biol Psychiatry 2013; 73: 1071–1077. [PMC free article][PubMed]
- Peña DF, Childs JE, Willett S, Vital A, McIntyre CK, Kroener S. Vagus nerve stimulation enhances extinction of conditioned fear and modulates plasticity in the pathway from the ventromedial prefrontal cortex to the amygdala. Front Behav Neurosci 2014; 8: 327. [PMC free article] [PubMed]
- Liberzon I, Krstov M, Young EA. Stress-restress: effects on ACTH and fast feedback. Psychoneuroendocrinology 1997; 22: 443–453. [PubMed]
- Yamamoto S, Morinobu S, Fuchikami M, Kurata A, Kozuru T, Yamawaki S. Effects of single prolonged stress and D-cycloserine on contextual fear extinction and hippocampal NMDA receptor expression in a rat model of PTSD. Neuropsychopharmacology 2008; 33: 2108–2116. [PubMed]
- Knox D, George SA, Fitzpatrick CJ, Rabinak CA, Maren S, Liberzon I. Single prolonged stress disrupts retention of extinguished fear in rats. Learn Mem 2012; 19: 43–49. [PMC free article][PubMed]
- Keller SM, Schreiber WB, Staib JM, Knox D. Sex differences in the single prolonged stress model. Behav Brain Res 2015; 286: 29–32. [PubMed]
- Childs JE, Alvarez-Dieppa AC, McIntyre CK, Kroener S. Vagus nerve stimulation as a tool to induce plasticity in pathways relevant for extinction learning. J Vis Exp 2015; 102: 53032. [PMC free article][PubMed]
- George MS, Sackeim HA, Rush AJ, Marangell LB, Nahas Z, Husain MM et al. Vagus nerve stimulation: a new tool for brain research and therapy. Biol Psychiatry 2000; 47: 287–295. [PubMed]
- Blanchard RJ, Blanchard DC. Passive and active reactions to fear-eliciting stimuli. J Comp Physiol Psychol 1969; 68: 129–135. [PubMed]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods 1985; 14: 149–167. [PubMed]
- De Boer SF, Koolhaas JM. Defensive burying in rodents: ethology, neurobiology and psychopharmacology. Eur J Pharmacol 2003; 463: 145–161. [PubMed]
- Mortolato M, Godar SC, Davarian S, Chen K, Shih JC. Behavioral disinhibition and reduced anxiety-like behaviors in monoamine oxidase B deficient mice. Neuropsychopharmacology 2009; 34: 2746–2757. [PMC free article] [PubMed]
- Njung’e K, Handley SL. Evaluation of marble-burying behavior as a model of anxiety. Pharmocol Biochem Behav 1991; 38: 63–67. [PubMed]
- Davis M, Falls WA, Campeau S, Kim M. Fear-potentiated startle: a neural and pharmacological analysis. Behav Brain Res 1993; 58: 175–198. [PubMed]
- Morgan CA, Grillon C, Southwick SM, Davis M. Fear-potentiated startle in posttraumatic stress disorder. Biol Psychiatry 1995; 38: 378–385. [PubMed]
- Takahashi T, Morinobu S, Iwamoto Y, Yamawaki S. Effect of paroxetine on enhanced contextual fear induced by single prolonged stress in rats. Psychopharmacology 2006; 2: 165–173. [PubMed]
- Hassert DL, Miyashita T, Williams CL. The effects of peripheral vagal nerve stimulation at a memory-modulating intensity on norepinephrine output in the basolateral amygdala. Behav Neurosci 2004; 118: 79–88. [PubMed]
- Manta S, Dong J, Debonnel G, Blier P. Enhancement of the function of rat serotonin and norepinephrine neurons by sustained vagus nerve stimulation. J Psychiatry Neurosci 2009; 34: 272–280. [PMC free article] [PubMed]
- Roosevelt RW, Smith DC, Clough RW, Jensen RA, Browning RA. Increased extracellular concentrations of norepinephrine in cortex and hippocampus following vagus nerve stimulation in the rat. Brain Res 2006; 1119: 124–132. [PMC free article] [PubMed]
- Kilgard MP. Harnessing plasticity to understand learning and treat disease. Trends Neurosci 2012; 35: 715–722. [PMC free article] [PubMed]
- Hulsey DR, Riley JR, Loerwald KW, Rennaker RL, Kilgard MP, Hays SA. Parametric characterization of neural activity in the locus coeruleus in response to vagus nerve stimulation. Exp Neurol 2016; 289: 21–30. [PMC free article] [PubMed]
- Engineer ND, Riley JR, Seale JD, Vrana WA, Shetake HA, Sudanagunta SP et al. Reversing pathological neural activity using targeted plasticity. Nature 2011; 470: 101–106. [PMC free article][PubMed]
- Hays SA, Khodaparast N, Sloan AM, Fayyaz T. The bradykinesia assessment task: an automated method to measure forelimb speed in rodents. J Neurosci 2013; 214: 52–61. [PubMed]
- Khodaparast N, Hays SA, Sloan AM, Hulsey DR, Ruiz A, Pantoja M et al. Vagus nerve stimulation during rehabilitative training improves forelimb strength following ischemic stroke. Neurobiol Dis2013; 60: 80–88. [PubMed]
- Porter BA, Khodaparast N, Fayyaz T, Cheung RJ. Repeatedly pairing vagus nerve stimulation with a movement reorganizes primary motor cortex. Cereb Cortex 2012; 22: 2365–2374. [PubMed]
- Shetake JA, Engineer ND, Vrana WA, Wolf JT. Pairing tone trains with vagus nerve stimulation induces temporal plasticity in auditory cortex. Exp Neurol 2012; 233: 342–349. [PubMed]
- Alvarez-Dieppa AC, Griffin K, Cavalier S, McIntyre CM. Vagus nerve stimulation enhances extinction of conditioned fear in rats and modulates Arc protein, CaMKII, and GluN2B-containing NMDA receptors in the basolateral amygdala. Neural Plast 2016; 1–11. [PMC free article] [PubMed]
- Jovanovic T, Kazama A, Bachevalier J, Davis M. Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology 2012; 62: 695–704. [PMC free article] [PubMed]
- Norrholm SD, Jovanovic T, Olin IW, Sands LA, Karapanou I, Bradley B et al. Fear extinction in traumatized civilians with posttraumatic stress disorder: relation to symptom severity. Biol Psychiatry2011; 69: 556–563. [PMC free article] [PubMed]
- Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology 2011; 36: 529–538. [PMC free article] [PubMed]
- Marek R, Strobel C, Bredy TW, Sah P. The amygdala and medial prefrontal cortex: partners in the fear circuit. J Physiol 2013; 591: 2381–2391. [PMC free article] [PubMed]
- O’Keane V, Dinan TG, Scott L, Corcoran C. Changes in hypothalamic-pituitary-adrenal axis measures after vagus nerve stimulation therapy in chronic depression. Biol Psychiatry 2005; 58: 963–968.[PubMed]
- Porges SW. The polyvagal theory: new insights into adaptive reactions of the autonomic nervous system. Cleve Clin J Med 2009; 76(Suppl 2): S86–S90. [PMC free article] [PubMed]
- Higgins CB, Vatner SF, Braunwald E. Parasympathetic control of the heart. Pharmacol Rev 1973; 25: 119–155. [PubMed]
- Furmaga H, Shah A, Frazer A. Serotonergic and noradrenergic pathways are required for the anxiolytic-like and antidepressant-like behavioral effects of repeated vagal nerve stimulation in rats. Biol Psychiatry 2011; 70: 937–945. [PubMed]
- George MS, Ward HE, Ninan PT, Pollack M, Nahas Z, Anderson B et al. A pilot study of vagus nerve stimulation (VNS) for treatment-resistant anxiety disorders. Brain Stimul 2008; 1: 112–121. [PubMed]
- Anderson P, Jacobs C, Rothbaum BO. Computer-supported cognitive behavioral treatment of anxiety disorders. J Clin Psychol 2004; 60: 253–267. [PubMed]
- Bowers ME, Ressler KJ. Interaction between the cholecystokinin and endogenous cannabinoid systems in cued fear expression and retention. Neuropsychopharmacology 2015; 40: 688–700. [PMC free article] [PubMed]
- Foa EB. Psychosocial treatment of posttraumatic stress disorder. J Clin Psychiatry 2000; 61: 43–48.[PubMed]
- Englot DJ, Chang EF, Auguste KI. Vagus nerve stimulation for epilepsy: a meta-analysis of efficacy and predictors of response. J Neurosurg 2011; 115: 1248–1255. [PubMed]
Vagal tone: a physiologic marker of stress vulnerability.
Abstract
Vagal tone is proposed as a novel index of stress vulnerability and reactivity with applications in all branches of medicine, and with particular value in pediatrics. The paper proposes a model emphasizing the role of the parasympathetic nervous system and particularly the vagus nerve in mediating homeostasis and defining stress. Measurement of cardiac vagal tone is proposed as a method to assess on an individual basis both the stress response and the vulnerability to stress. The method monitors the neural control of the heart via the vagus (ie, vagal tone) as an index of homeostasis. The method provides a standard instrument with statistical parameters that are comparable between patients and throughout the life span. This noninvasive method will allow the assessment of the stressful impact of various clinical treatments on the young infant and permit the identification of individuals with vulnerabilities to stress
The polyvagal perspective.
Abstract
The polyvagal theory introduced a new perspective relating autonomic function to behavior, that included an appreciation of the autonomic nervous system as a “system,” the identification of neural circuits involved in the regulation of autonomic state, and an interpretation of autonomic reactivity as adaptive within the context of the phylogeny of the vertebrate autonomic nervous system. The paper has two objectives: first, to provide an explicit statement of the theory; and second, to introduce the features of a polyvagal perspective. The polyvagal perspective emphasizes how an understanding of neurophysiological mechanisms and phylogenetic shifts in neural regulation leads to different questions, paradigms, explanations, and conclusions regarding autonomic function in biobehavioral processes than peripheral models. Foremost, the polyvagal perspective emphasizes the importance of phylogenetic changes in the neural structures regulating the autonomic nervous system and how these phylogenetic shifts provide insights into the adaptive function and the neural regulation of the two vagal systems.
- PMID:
- 17049418
- PMCID:
- PMC1868418
- DOI:
- 10.1016/j.biopsycho.2006.06.009