This is an educational article and contains a collection of information from various sources. This article contains a brief description of terms I often use, it exists to assist my clients who are interested in doing some research. If you have questions please contact me.
Before we discuss the Hypothalamus we will quickly define “Homeostasis”. Homeostasis is the process by which a steady state of equilibrium, or constancy, in the body with respect to physiological functions and chemical compositions of fluids and tissues is maintained.
Physiological set points refer to the baseline level at which functions such as heart rate, and at which chemical compositions such as plasma sodium concentration are normally maintained.
These set points are represented in the brain by specific discharge rates in neurons dedicated to the monitoring and control of specific physiological processes. Thus, separate groups of neurons are dedicated to the control of heart rate, temperature, etc., by their set point discharge rate.
The hypothalamus has the greatest concentration of nuclei at which set points are encoded, monitored and controlled, and so can be considered as the key brain region for the control of homeostasis.
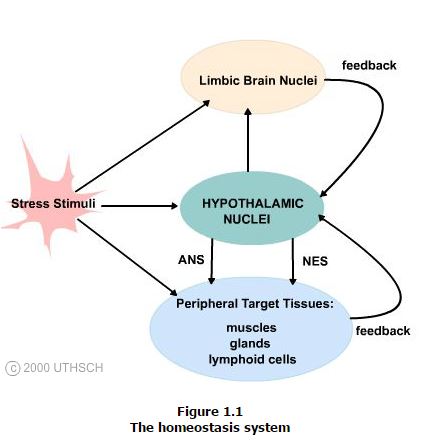
Specific receptors and sensors throughout the body detect disruptions in the normal balance of body functions and chemistry that are produced by stress stimuli that can range from injury or infection to pain and emotional distress. These data are transmitted to the central nervous system and affect the discharge rate of set point neurons in hypothalamic nuclei.
These changes in discharge rate result in altered hypothalamic efferent (outwardly) outflow and hence change in the functions of regulatory systems that counteract the stress stimulus and restore homeostasis. These effects include alterations in the functions of the autonomic nervous system (ANS), endocrine and immune systems, as well as alterations in behavior by hypothalamic influences on limbic brain circuitry. Each of the target systems influenced by the hypothalamus return feedback controls onto the hypothalamus completing a circuit and so establishing a homeostasis system.
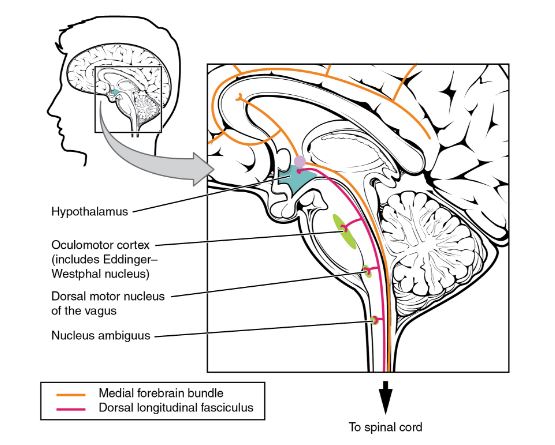
The hypothalamus is the source of most of the central control of autonomic function. It receives input from cerebral structures and projects to brain stem and spinal cord structures to regulate the balance of sympathetic and parasympathetic input to the organ systems of the body. The main pathways for this are the medial forebrain bundle and the dorsal longitudinal fasciculus.
These two tracts connect the hypothalamus with the major parasympathetic nuclei in the brain stem and the preganglionic (central) neurons of the thoracolumbar spinal cord.
The hypothalamus also receives input from other areas of the forebrain through the medial forebrain bundle. The olfactory cortex, the septal nuclei of the basal forebrain, and the amygdala project into the hypothalamus through the medial forebrain bundle. These forebrain structures inform the hypothalamus about the state of the nervous system and can influence the regulatory processes of homeostasis.
A good example of this is found in the amygdala, which is found beneath the cerebral cortex of the temporal lobe and plays a role in our ability to remember and feel emotions.
1.1 Anatomy of the Hypothalamus
The role of the hypothalamus in regulation of homeostasis is essential for survival and reproduction of the species. The importance of this function is underscored by the structural organization and connectivity of the hypothalamus as almost every major subdivision of the neuraxis communicates with the hypothalamus and is subject to its influence.
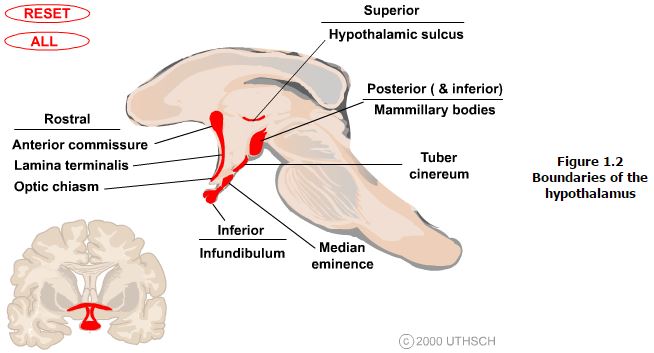
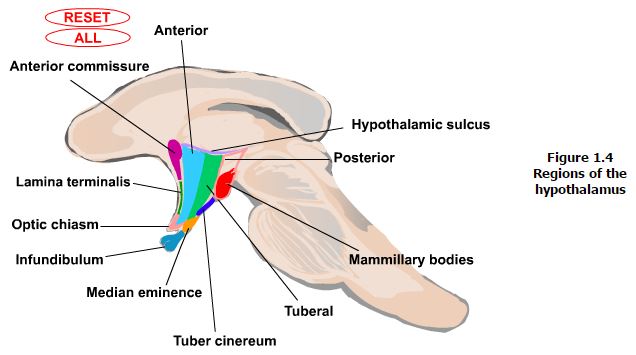
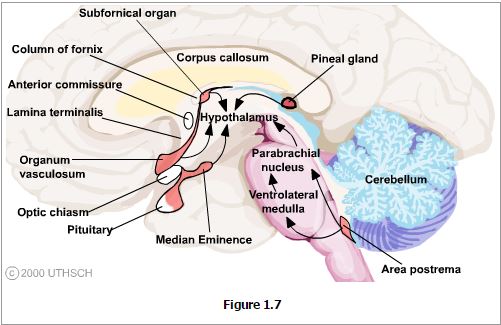
Landmarks that are visible on the ventral and medial (ventricular) surfaces of the brain define the boundaries of the hypothalamus. The rostral boundary visible on the ventral surface of the brain is formed by the optic chiasm while the mammillary bodies define the posterior boundary. Between these structures the oval prominence from the floor of the third ventricle is the tuber cinereum and evaginating from this is the median eminence which then tapers into the infundibular stalk which together form the inferior boundary of the hypothalamus. On the medial (ventricular) surface of the brain other structures contributing to the rostral boundary that are visible include the lamina terminalis and the anterior commisure. Also visible on the medial surface of the brain is the hypothalamic sulcus, which is the rostral continuation of the sulcus limitans that defines the superior boundary of the hypothalamus. Finally, the internal capsule that is only visible on coronal or horizontal sections of the brain forms the lateral boundary.
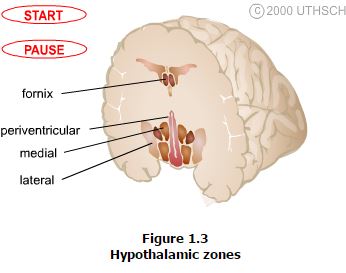
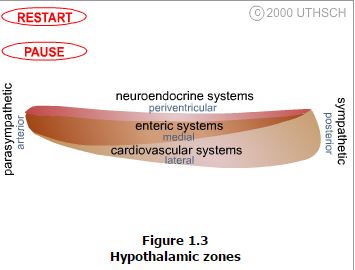
The hypothalamus is composed of three longitudinally oriented cell columns, or zones, that run the entire rostrocaudal length of the hypothalamus (Figure 1.2). These zones can be further subdivided into four nuclear groups, or regions, based on rostrocaudal position.
Zones. Immediately bordering the third ventricle, just inside of the ependymal cell lining, is a thin layer of cells that comprise the periventricular zone. This zone contains few distinct nuclei, but two that are very prominent are the arcuate nucleus and the paraventricular nucleus, which are involved in neuroendocrine and autonomic regulation. Immediately adjacent to the periventricular zone is the medial zone, which is comprised of several cytoarchitectonically distinct nuclei that are listed below. Nuclei in the medial zone are especially involved in the regulation of the autonomic nervous system as well as involved in regulation of the neuroendocrine system. Finally, the lateral zone, has few nuclei or clear landmarks, but contains important fiber pathways such as the median forebrain bundle. Demarcated by the fornix, the lateral zone is involved in regulation of the autonomic nervous system.
Regions. Each of the zones described above are further subdivided into regions based on rostrocaudal landmarks (Figure 1.4). The anterior region runs from the lamina terminalis to the caudal aspect of the optic chiasm. The portion of the anterior region that is rostral to the optic chiasm is often also referred to as the preoptic region, however this distinction is now less emphasized. The next region that is identified when proceeding caudally is the tuberal region. The margins of this region include the areas that are above and including the tuber cinereum. Finally, the posterior region is defined by the area above and including the mammillary bodies.
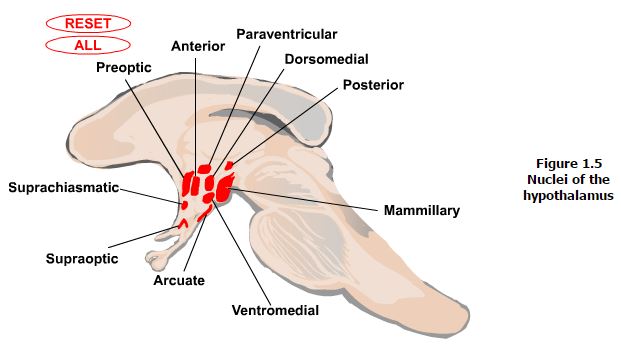
Nuclei. There are eleven major nuclei in the hypothalamus (Figure 1.5). The functions of many of these will be covered in further detail in later sections. However, a brief note on the organization of these can be useful. The nuclei can be grouped based on their locations in the hypothalamic zones and regions. Starting medially, the paraventricular nucleus is located in the periventricular zone and runs rostro-caudally through the anterior into the tuberal region. The arcuate nucleus also has a portion located in the periventricular zone though it also extends laterally into the medial zone. This nucleus sits in the floor of the tuberal region of the hypothalamus. Both of these nuclei, along with the supraoptic nucleus, located just above the optic chiasm in the anterior region of the medial zone extending laterally into the lateral zone, have key roles in neuroendocrine regulation. The paraventricular nucleus also has an important role in regulation of the autonomic nervous system. Additional nuclei found in the anterior region of the medial zone include the suprachiasmatic nucleus involved in circadian timing, the anterior nucleus involved in control of the autonomic nervous system, and the preoptic nucleus which also extends into the lateral zone and involved in control of the autonomic nervous system. Additional nuclei in the tuberal region of the medial zone include the dorsomedial and ventromedial nuclei, which are involved in control of behavior and of appetite, body weight and insulin secretion, respectively. Nuclei of the posterior region of the medial zone include the posterior nucleus, which is another autonomic nervous system control center, and the mammillary nuclei, which are involved in control of emotional expression and memory. Finally, the lateral tuberal complex in the tuberal region of the lateral zone is involved in control of appetite.
1.2 Circuitry of the Hypothalamus
The hypothalamus has the most complex circuitry of any brain region. Like other brain areas there are neural interconnections. But unlike other brain areas, there are also extensive non-neural communication pathways between the hypothalamus and other brain regions and the periphery.
Neural Connections. The most noteworthy (and complex) feature of the neural connections of the hypothalamus is that except for a few exceptions, they are extensively bi-directional.
Limbic Circuits. These pathways are essential for the normal expression and control of emotions, learning and reproductive behavior. The bi-directional (afferent and efferent) pathways include the medial forebrain bundle, the fornix, the stria terminalis and the ventral amygdalofugal pathway. The medial forebrain bundle interconnects basal forebrain structures including the septal nuclei and ventral striatum with hypothalamus and structures in the brainstem tegmentum including the locus ceruleus, the parabrachial nucleus, dorsal motor nucleus of the vagus.
The fornix interconnects the hippocampal formation to the septal, preoptic and medial mammillary nuclei. The stria terminalis interconnects the amygdala to the septal region and the hypothalamus especially, the preoptic and ventromedial regions. Finally, the ventral amygdalofugal pathway interconnects the amygdala, especially the central amygdaloid nucleus with the septal region and the preoptic areas of the hypothalamus.
In addition to these bi-directional pathways, there are also two unidirectional efferent limbic pathways from the hypothalamus. The mammillo-thalamic tract projects from the mammillary nuclei to the anterior nucleus of the thalamus. The anterior nucleus of the thalamus in turn projects to the cingulate cortex, which completes the circuit of Papez by projecting back onto the subiculum of the hippocampus. The circuit of Papez was the first circuit proposed to mediate emotions and still is considered one of the chief circuits of the limbic system. The mammillo-tegmental tract projects from the mammillary nuclei to the brainstem tegmentum and as far caudal as the lateral gray of the spinal cord.
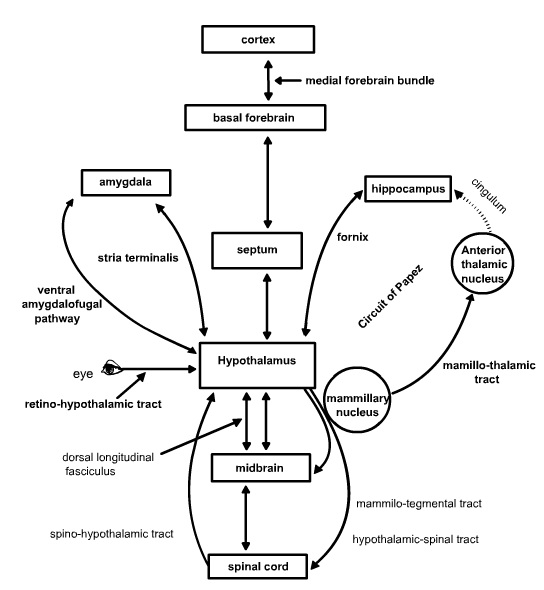
Sensory and Autonomic Circuits. These pathways provide visceral and somatosensory input to the hypothalamus and output of the hypothalamus to control the autonomic nervous system. These pathways are especially important for the control of feeding, insulin release and reproduction. The bi-directional pathways in this circuitry include the medial forebrain bundle noted as part of limbic circuitry above, as well as the dorsal longitudinal fasciculus. Whereas the medial forebrain bundle runs laterally through the brainstem and hypothalamus, the dorsal longitudinal fasciculus runs medially through the periventricular and periaqueductal gray matter. Both pathways bring visceral and somatic input to the hypothalamus from the nucleus of the solitary tract, the parabrachial nuclei, the reticular formation and the periaqueductal gray. The medial forebrain bundle also brings monoaminergic fibers containing noradrenaline and serotonin into the hypothalamus from various brainstem nuclei including the raphe nuclei that have key roles in modulating neuroendocrine functions. More rostral projections of these monoaminergic fibers as well as peptide-containing efferent fibers that originate in the hypothalamus and join the medial forebrain bundle as it ascends into the orbital cortex, insula and frontal cortex are involved in the control of motivation. Descending efferent projections of the hypothalamus through these pathways terminate on parasympathetic nuclei of the brainstem such as the dorsal motor nucleus of the vagus. Unidirectional afferent input to the hypothalamus is derived from the spinohypothalamic tract and the retino-hypothalamic tract. The spinohypothalamic tract is a component of the anterolateral system of somatosensory fibers that also includes the spinothalamic tract and provides input concerning pain as well as input necessary for orgasm. The retino-hypothalamic tract provides input to the suprachiasmatic nucleus that is used to entrain circadian rhythms to the light-dark cycle. Finally, unidirectional efferent pathways from the hypothalamus include the hypothalamo-spinal tract, which projects onto brainstem and finally spinal preganglionic sympathetic and parasympathetic neurons in the spinal intermediolateral cell column, and a histamine projection to thalamus and cortex from the inferior lateral tuberal region that regulates the sleep-wake cycle.
Neuro-Humoral Connections. Unlike any other brain structure the hypothalamus both sends and receives information by way of the blood stream. There are two pathways that comprise the neuro-humoral connections of the hypothalamus.
The Pituitary. These pathways include the hypophyseal-portal system of blood vessels that surround the median eminence, the infundibulum and pituitary gland. The details of this system in neuroendocrine function will comprise the third chapter of this section.
Circumventricular Organs. There are several sites at which the blood brain barrier is highly permeable and at which specific transporters are present that allow passage of chemosensory stimuli from the blood into the brain. For example, the organum vasculosum of the lamina terminalis is the site at which pyrogens such as interleukin-1 and tumor necrosis factor bind to receptors that transport these molecules into the CNS and initiate the central synthesis of prostaglandins. These in turn act on the anterior nucleus to initiate a change in body temperature set-point resulting in fever. Passage of hormones through both the organum vasculosum and the median eminence is essential for normal feedback on the hypothalamus for neuroendocrine control. The area postrema is the location of the chemotoxic trigger zone at which emesis is induced by various toxins in the blood stream and that affect the hypothalamus to induce taste aversion. Passage of peptides through the subfornical organ are thought to participate in mechanisms of learning, while passage of signals through the pineal body affects circadian and circannual timing patterns.
1.3 Functions of the Hypothalamus
It has been highlighted several times in this section that the overarching function of the hypothalamus is the integration of body functions for the maintenance of homeostasis. The multiplicity of functions that are entailed in this level of integration should be intuitively obvious. The table below lists many of these functions and the nuclear groups that are most closely associated their execution.
| Nucleus | Zone(s) | Region(s) | Functions |
| Paraventricular | Periventricular, Medial | Anterior,Tuberal | Fluid balance, milk let-down, parturition, autonomic & anterior pituitary control |
| Preoptic | Medial, Lateral | Anterior | Lateral anterior thermoregulation, sexual behavior |
| Anterior | Medial | Anterior | Lateral anterior thermoregulation, sexual behavior |
| Suprachiasmatic | Medial | Anterior | Biological rhythms |
| Supraoptic | Medial, Lateral | Anterior | Fluid balance, milk let-down, parturition |
| Dorsomedial | Medial | Tuberal | Emotion (rage) |
| Ventromedial | Medial | Tuberal | Appetite, body weight, insulin regulation |
| Arcuate | Periventricular, Medial | Tuberal | Control of anterior pituitary, feeding |
| Posterior | Medial | Posterior | Thermoregulation |
| Mammillary | Medial | Posterior | Emotion and short-term memory |
| Lateral Complex | Lateral | Tuberal | Appetite and body weight control |
Thermoregulation, Neuroendocrine control, Feeding and Satiety. The details concerning thermoregulation, neuroendocrine function, and control of feeding will be the subject of later chapters.
Biological Timing and Rhythms. Circadian timing refers to the daily fluctuations that occur in hormone levels, body temperature, sleep-wake cycle, etc.; while circannual timing refers to fluctuations in function that occur on a yearly cycle. The chief hypothalamic nucleus involved in this process is the suprachiasmatic nucleus (SCN), which can be considered as the body’s master clock. The neurons in the SCN have an intrinsic rhythm of discharge activity that will re-cycle in the absence of light at 25 hour intervals. This activity is an intrinsic property of SCN neurons that can be maintained for days while the cells are maintained in culture. Input to the SCN from the retinohypothalamic tract resets and entrains the activity of SCN neurons to the daily 24 hour light-dark cycle by regulating the transcription of the light-sensitive clock, bmal, period (per) and cryptochrome (cry) genes. The retino-hypothalamic tract is a non-rod, non-cone dependent input to the SCN from a subset of retinal ganglion cells that are directly activated by light interacting with the pigment melanopsin. The SCN has projections into multiple hypothalamic nuclei that control the specific functions that show daily or annual rhythms. Thus, the SCN is considered as a master pacemaker that regulates the functions of multiple intra- and extra-hypothalamic slave oscillators. One extraordinary example of an extra-hypothalamic slave oscillator is the induction of fetal circadian timing from the mother. A specific, and perhaps more concrete example of this circuitry is illustrated by the regulation of melatonin secretion. Activation of the SCN by light results in increased input to the paraventricular nucleus, which in turn activates sympathetic pre-ganglionic neurons in the T1-T2 spinal intermediolateral cell column. These neurons inhibit the superior cervical ganglion which sends noradrenergic innervation into the pineal gland that inhibits the release of melatonin. With the onset of darkness, this inhibition is removed, and so melatonin secretion increases through a disinhibition process
There are two major classes of disorders in circadian timing, phase shifting and entrainment failure, both of which manifest themselves as sleep disorders. The most common phase shift disorder is the rapid time-zone change syndrome, or jet lag, characterized by daytime sleepiness and nighttime insomnia. The molecular biology of this disorder is becoming well-defined. Circadian disruption is more sensitive to advances in local time than to delays. Circadian expression of mPer in SCN reacts rapidly to an advance in light onset whereas expression of mCry advances slowly, at a maximum rate of 3hours/cycle. It is only when the expression of both genes resume their baseline parallel expression that the behavioral and light:dark cycles become re-aligned. In contrast, mPer and mCry expression cycles react rapidly and in parallel with a delay in light cycle, such that a complete reset is achieved within one cycle. A second type of phase shift disorder is delayed sleep phase syndrome commonly seen in adolescents and possibly linked to an endocrine-mediated desensitization of SCN pacemakers to phase-advancing stimuli. Finally, advanced sleep phase syndrome, characterized by onset of sleep in the early evening followed by very early pre-dawn awakening is commonly observed in the elderly and is associated with a missence mutation in mPer2. Entrainment failure is often, though not always, observed in the blind. It is important to remember that the retino-hypothalamic tract has nothing to do with vision and so can be preserved in the blind, and may also be absent in those with vision.
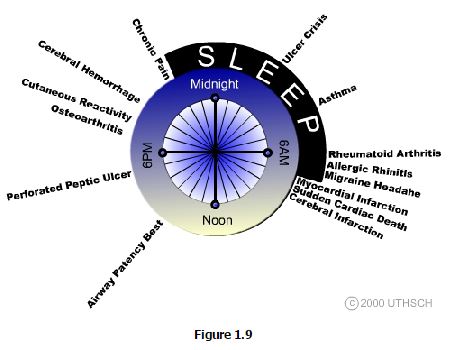
It has become increasingly evident that circadian timing can have tremendous impact on the susceptibility to disease as well as conversely, to the optimal timing of curative therapy (Figure 1.9). Chronomorbidity refers to the observation that certain disorders characteristically show peak prevalence at particular times of the day, whereas Chronotherapeutics is the application of therapies at the time of day when their effects can be expected to have the greatest impact. The best current example of effective chronotherapeutics is that treatment of seasonal affective disorder (a form of entrainment failure) is successfully treated with bright light therapy only when applied during the morning hours.
1.4 Summary
The hypothalamus is the key brain site for integration of multiple biologic systems to maintain homeostasis. Neurons in the hypothalamus discharge in relation to multiple physiologic indices and change discharge rate with changes in these indices, thus establishing set points. The three major systems controlled by the hypothalamus for maintenance of homeostasis are the autonomic nervous system, the neuroendocrine system, and the limbic system.
The hypothalamus has well defined anatomical boundaries. Different regions of the hypothalamus are especially associated with the control of specific physiological subsystems.
The broad scope of brain regions affected by the hypothalamus is reflected by a very widespread extent of connectivity of the hypothalamus to other brain areas and by unique neuro-humoral communication pathways.
One key function of the hypothalamus is regulation of body functions in concert with the daily light:dark cycle. Intrinsic timing mechanisms of neurons in the suprachiasmatic nucleus controlled by the expression of the light sensitive clock, bmal, per, and cry genes establish the master pacemaker of the body. The activity of these cells is set in phase to light by inputs from a special subset of non-rod, non-cone dependent melanopsin-containing retinal ganglion cells via the retino-hypothalamic tract.
Source: University of Texas, Health. Neuroscience Online. Chapter 1: Hypothalamus: Structural Organization http://nba.uth.tmc.edu/neuroscience/s4/chapter01.html
Find chapter 2 here: http://nba.uth.tmc.edu/neuroscience/s4/chapter02.html
Chapter 2 Summary
Secretion of the posterior pituitary hormones is directly from magnacellular neurons of the paraventricular and supraoptic nuclei into the circulation. These neurons project axons into the posterior pituitary via the hypothalamo-neurohypophyseal tract and terminate on a capillary bed of the inferior hypophyseal artery. Control of release in this system is under neural control and so this represents a reflex system with neural input and hormonal output.
Secretion of anterior pituitary hormones is driven by the influence of releasing- or release-inhibiting hormones (factors) that are synthesized in parvocellular neurons of the supraoptic, paraventricular, arcuate and periventricular hypothalamic nuclei. These neurons project axons in the tubero-infundibular tract onto a capillary bed of the superior hypophyseal artery at the base of the median eminence. These capillaries coalesce into the hypophyseal portal veins, which descend to the anterior pituitary and form a second capillary bed where the anterior pituitary hormones are released. The anterior pituitary hormones diffuse to peripheral targets to provoke release of endocrine hormones that induced tissue effects. Control of release in this system is via feedback of releasing-hormones, anterior pituitary hormones, and peripheral endocrine hormones onto hypothalamic and pituitary cells in a series of feedback loops. This system thus represents a hormone-and neural-evoked and hormone-output reflex.
The immune system provokes neural and neuroendocrine responses in an acute phase “sickness” response to illness or injury. The immune system is also influenced by neural activity.
Chapter 3 (parts of)
3.1 Defining the Central Autonomic Network
Because many students have been led to believe that the autonomic nervous system is relatively primitive, most have concluded that normal regulation of this system occurs at ganglionic, or at best, spinal levels. Thus, they are often quite surprised to discover that dysfunction of the brain is typically accompanied by autonomic dysfunction that can be life-threatening. For example, patients with spinal transection can have severe hypertensive crises provoked by a full bladder, impacted colon, or even stroking of the skin. This is not to say that the spinal cord and autonomic ganglia do not play important roles in autonomic regulation. But, that the organization of autonomic output takes place at supraspinal levels.
Extensive interconnection occurs between sites receiving visceral inputs and that control autonomic efferent outputs, between sites for the control of sympathetic versus parasympathetic nervous system output, and between sites for autonomic control and somatic, endocrine and limbic circuitry. Collectively, this set of interconnections is termed the central autonomic network.
3.2 Structure of the Central Autonomic Network
The central autonomic network is composed of both hypothalamic and extra-hypothalamic nuclei. Some of these sites regulate sympathetic outflow whereas others regulate parasympathetic outflow. This structure was first revealed in lesion studies that revealed multisynaptic connections descending from the hypothalamus and midbrain to preganglionic neurons in the brainstem and spinal cord. Similarly, connections from various limbic brain structures, most especially the amygdala, through the hypothalamus have been demonstrated. The net result of this network in full operation is the induction of autonomic responses to visceral and somatic stress stimuli, such as elevated heart rate and blood pressure with the onset of pain. Alternatively, chronic hypertension in type “A” or stressed individuals represents increased central autonomic outflow in response to increased limbic system input. Hierarchy in the autonomic network results in the loops from the brainstem to spinal cord being responsible for rapid short-term regulation of the autonomic nervous system, hypothalamic-brainstem-spinal cord pathways serving longer-term, metabolic and reproductive regulation, and finally limbic system-hypothalamic-brainstem-spinal cord loops serving anticipatory autonomic regulation.
- Hypothalamic Structures. The single most important hypothalamic nucleus of the central autonomic network is the paraventricular nucleus (PVN). The PVN has two morphological classes of neurons that fall into three functional categories. The first morphological class is comprised of magnacellular (big) neurons. These neurons contain vasopressin and oxytocin and project their axons into the posterior pituitary where these hormones are released directly into the blood stream. The second morphological class is comprised of parvocellular (small) neurons. The parvocellular PVN neurons also include a neuroendocrine-related functional subset that project to the median eminence and secrete releasing hormones into the hypophyseal portal blood stream for control of anterior pituitary hormone secretion. More on these two functional groups will be covered in the next chapter. Finally, a group of parvocellular neurons comprise the third functional group of PVN neurons with these involved in central autonomic control.There are three types of pre-autonomic parvocellular neurons (Types A, B and C) separable based on anatomical and physiological criteria, as well as based on subnuclear location within the PVN. Pre-autonomic PVN neurons project directly onto preganglionic autonomic neurons in the dorsal motor nucleus of the vagus, the autonomic relay nuclei of the brainstem (A5, rostral ventral lateral medulla) and even directly to the intermediolateral spinal columns. These projections descend ipsilaterally through the brainstem and spinal cord with four points of decussation (supramammillary, pontine tegmentum, commisural part of the nucleus of the solitary tract (the major one), lamina X of the spinal cord) so that ultimately innervation is bilateral but with an ipsilateral dominance. Thus, the PVN, unlike any other brain site, has direct influence over both sympathetic and parasympathetic outflow. Furthermore, the PVN receives direct sympathetic and parasympathetic afferent inputs from trigeminal pars caudalis (sympathetic) and the nucleus of the solitary tract (parasympathetic). The PVN therefore is the only brain site in a closed efferent-afferent reflex loop with both the sympathetic and parasympathetic nervous systems.Other hypothalamic nuclei in the central autonomic network include the dorsomedial nucleus, the lateral hypothalamic area, the posterior hypothalamic nucleus and the mammillary nucleus. These nuclei send and receive projections from the PVN, the dorsal motor nucleus of the vagus, the central gray matter, the parabrachial nucleus, the nucleus of the solitary tract, the lateral and ventral medulla and the intermediolateral spinal columns. The lateral hypothalamus is especially involved in cardiovascular control as well as in control of feeding, satiety and insulin release.
- Extra-hypothalamic Structures. Numerous brain structures were itemized above as innervation targets of the hypothalamic structures of the central autonomic network. These extra-hypothalamic sites can be roughly divided into those associated with control of the two components of the autonomic nervous system. The sites associated with control of sympathetic outflow include the norepinephrine-containing neurons of the dorsal mesencephalon (locus ceruleus) and the rostral and caudal ventrolateral medulla (the A5 and A1 regions) and the serotonin-containing neurons of the pontine and medullary raphe nuclei. The extra-hypothalamic sites associated with control of parasympathetic outflow include the central nucleus of the amygdala, the dorsal motor nucleus of the vagus, the nucleus ambiguus, the raphe nuclei, the periaqueductal gray, and the parabrachial nucleus. Finally, limbic cortices, including the cingulate, orbitofrontal, insular and rhinal cortices, and the hippocampus influence both sets of autonomic outflow.
3.4 Disorders of the Central Autonomic Control
- Autonomic Dysreflexia is a condition observed in about 85 percent of patients following spinal cord injury above C6. Exaggerated autonomic reflexes, especially sudden dramatic increases in blood pressure are provoked by inappropriate stimuli, such as pressure on the bladder.
- Riley-Day Syndrome (familial dysautonomia) is an autosomal recessive disorder in Ashkenazi Jews associated with decreased tearing and sensitivity to pain and absent fungiform papillae on the tongue. Episodic abdominal crises and fever are very common as is orthostatic hypotension.
- Shy-Drager Syndrome is a progressive degenerative condition of unknown origin affecting cells of the central autonomic network in the brainstem, intermediolateral cell column, locus ceruleus, dorsal motor nucleus of the vagus, and other nuclei including the substantia nigra caudate nucleus, and cerebellum. The presence of Lewy bodies in many of these areas suggests this syndrome may be related to Parkinson’s disease, in which there is also often a high degree of autonomic dysfunction. The hallmark sign is profound orthostatic hypotension without a compensatory increase in heart rate.
- Sudden Infant Death Syndrome is thought to be a developmental defect in the central autonomic network of the brainstem involved with respiratory drive. An abrupt increase in facial skin temperature related to the onset of periods of apnea suggest there may be a broader developmental defect of central autonomic control.
- Horner’s Syndrome typically results following damage to the dorsolateral pons or medulla and is characterized by a profound disturbance in sympathetic nervous system function. A common cause of this type of lesion is thrombosis of the posterior inferior cerebellar artery or following damage to the white matter of the cervical spinal cord where the hypothalamic-spinal tract descends. The most common signs in Horner’s syndrome are ipsilateral miosis, ptosis, anhidrosis and erythema.
.5 The Central Autonomic Network and Control of Body Temperature
As noted above, the central autonomic network consists of three hierarchically ordered circuits or loops: the short-term brainstem-spinal loops, and the limbic brain-hypothalamic-brainstem-spinal cord loops mediating anticipatory and stress responses, and the intermediate length hypothalamic-brainstem-spinal cord loops mediating longer-term autonomic reflexes. Here we focus on this later loop in its role in thermoregulation. A later chapter will focus on this loop in the regulation of feeding.
The Hypothalamic Basis of Temperature Set-Point. Regulation of core temperature is essential because most of the metabolic processes necessary for life are strongly temperature-dependent. The normal body temperature set-point is primarily determined by the activity of neurons in the medial preoptic and anterior hypothalamic nuclei as well as by neurons in the adjoining medial septal nuclei. Collectively this region is often termed the preoptic anterior hypothalamus (POAH). The second region that also plays a critical, though subservient role to the POAH, in temperature regulation is the posterior hypothalamus.
3.9 Summary
Several forebrain, diencephalic and brainstem structures are interconnected to organize the output of the autonomic nervous system. Collectively, this is referred to as the central autonomic network and is further organized into a hierarchy of functional loops.
The hypothalamus is the key brain site for central control of the autonomic nervous system, and the paraventricular nucleus is the key hypothalamic site for this control. The major pathway from the hypothalamus for autonomic control is the dorsal longitudinal fasciculus.
Regulation of body temperature is one example of hypothalamic control of brainstem and spinal autonomic nuclei related to longer-term autonomic reflexes. Thermoregulation is principally a function of warm-sensitive neurons of the preoptic anterior hypothalamus that directly control the dissipation of heat.
Fever is the most common disorder of thermoregulation. Fever is following the release of endogenous pyrogens that elevate the level of prostaglandin E2 in the preoptic anterior hypothalamus, which causes a decrease in activity of warm-sensitive neurons and subsequent disinhibition of cool-sensitive neurons.
Chapter 4 – Central Control of Feeding Behavior
Feeding by its nature is intermittent, yet the need for energy in tissues is constant. Thus, mechanisms have evolved for the ebb and flow of nutrients after feeding and during the post-absorptive period; and the maintenance of near-normal function during fasting. The remarkable stability of body weight in persons with access to adequate food supplies is testament to the precision by which metabolic needs are monitored and maintained. Aberrations in these controls can produce serious and even life-threatening conditions.
4.1 Theories of Caloric Homeostasis
Two major hypotheses have been put forward to account for the process by which the usual balance in caloric intake is established.
 |
|
Figure 4.2 |
The Depletion-repletion Hypothesis. The depletion-repletion hypothesis is essentially based on the idea of a caloric set-point. In its simplest version individuals match energy expenditure and energy intake without reference to the level of fat storage. In more complicated models fat (energy) depots, and presumably other nutrient depots, send signals to the brain about their current status. These inputs are compared to desired set points and food intake and energy expenditure are modified to effect adjustments to the levels of stores. The best evidence for this view is from small mammals. Glucose-responsive neurons that increase firing rate with glucose levels and glucose-sensitive neurons that are stimulated as glucose levels fall have been identified. Further, small mammals will habitually return to a body mass that is appropriate for their age, stage of development and/or environment after a forced perturbation. Thus, rats exposed to a period of food restriction or imposed overfeeding (gavage) express a compensatory hyper- or hypophagia on return to ad libitum feeding. The current studies on leptin (see below) has heightened interest in this potential mechanism.
The Primed Response Hypothesis. This hypothesis essentially boils down to the idea that animals will eat whenever an opportunity presents itself unless it is specifically inhibited. Evidence in support of this idea comes from studies examining the relationship between caloric content in meals and feeding intervals. There is not a correlation between the amount eaten in a meal and the interval to the previous meal. Rather, the larger a given meal, the longer it will be to the next. This suggests that eating is inhibited by satiety signals generated in response to a meal.
4.2 Mechanisms of Satiety
There are two main issues in the concept of satiety. The first concerns the mechanisms that contribute to the termination of feeding at a given meal, whereas the second concerns the mechanisms that govern interprandial intervals. These mechanisms overlap to a degree.
Neural Signals are essentially dependent on the vagus and involve both afferent and efferent components. The afferent signals in the vagus conveyed to the brain that function to limit meal size include information from stretch receptors in the stomach wall, and sensors in the portal blood vessels for cholecystokinin (CCK), glucose, osmolality and pH. All these stimuli limit meal size. Vagal afferents synapse in the nucleus of the solitary tract. In addition, the vagus sends afferents to the area postrema. These sites send projections to the paraventricular nucleus to inhibit feeding. The vagus also functions in an afferent capacity in that input from the vagus to the pancreas mediates the cephalic phase of insulin release. The sight and smell of food initiates activity in limbic cortex that in turn activates the central autonomic network. Outflow to the dorsal motor nucleus of the vagus and finally to the pancreas results in the first surge of insulin in the blood that accompanies a meal. Two additional increases in plasma insulin later occur as food enters the gut (the gastrointestinal phase) and then as nutrients enter the intestines (the substrate phase). In fact, these stimuli also result in satiety as insulin not only promotes energy storage but also acts as a humoral signal back to the brain for satiety.
Humoral Signals for satiety are an extremely hot topic of research due to the vast potential weight-control market. The main factors in the blood that affect the CNS to limit/promote feeding are insulin, leptin, glucose, and CCK. Insulin, leptin and glucose gain access to the CNS at the circumventricular organs of the median eminence and the area postrema and act on specific receptors in neurons of the nuclear cell groups adjoining these areas. Insulin and leptin have received considerable recent interest as the mediators of satiety signals related to the caloric content of a meal and in relation to maintenance of a body weight set point, respectively. Chief among the CNS sites of action for these compounds as discussed below is the arcuate nucleus. CCK exerts its chief effects on satiety in the periphery by an action on the vagus to potentiate the responses to glucose, pH, and osmolality in the portal blood. CCK derived from neurons in the hypothalamus is also a transmitter substance in the CNS satiety network (see below).
4.3 The CNS Satiety Network
|
Figure 4.3 |
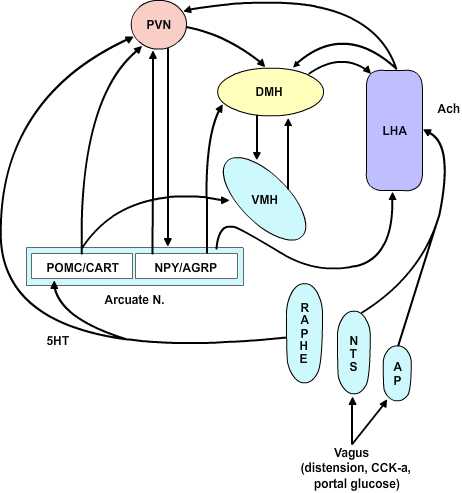
The Dual Center Hypothesis evolved about fifty years ago when it was demonstrated that bilateral lesions placed in the region of the ventromedial hypothalamus (VMH) produced a condition of voracious appetite and resulting marked hyperphagia. These animals would ultimately become remarkably obese. On the other hand, bilateral lesions of the ventrolateral hypothalamus (VLH) produced an anorexic condition that resulted in animals failing to feed and ultimately wasting. The assumption behind this set of experiments was that there are specific feeding and satiety centers. The conclusion in this set of experiments was that the feeding center was the VLH and the satiety center was the VMH. Although this dual center hypothesis has proven useful, the CNS satiety network has proven to be more complex. More recent repetitions of the earlier lesion studies showed that in fact animals with VMH lesions would not eat continually without limit, nor would the animals with VLH lesions necessarily starve to death. Rather, the VMH lesioned animals would eat excessively until they reached some new higher body weight at which point they would then reduce their food consumption to maintain this new set point. Likewise, once the VLH lesioned animals had dropped to some new level, they would again eat normally to maintain the new lower body weight set-point. These studies indicate that satiety and feeding is a balancing process between two major groups of neurochemical pathways that ultimately govern the relative tone between the sympathetic and parasympathetic components of the central autonomic network. The main entry points for information to this network are both neural and humoral. The neural inputs are from the vagus nerve via synapses in the nucleus of the solitary tract and the area postrema as well as inputs from the raphe nuclei. These inputs ascend in the medial forebrain bundle to the paraventricular and the lateral hypothalamic area (LHA). The humoral inputs pass the circumventricular organs and affect the area postrema and the arcuate nucleus. The arcuate nucleus projects into the LHA and the ventromedial nucleus as well as to the paraventricular and dorsomedial (DM) nucleus. The paraventricular nucleus returns inputs to the arcuate nucleus as well as to the VMH and LHA. There are no direct interconnections between the VMH and LHA, rather these are processed through the DM.
 |
|
Figure 4.4 |
Neurochemistry. Several peptides are involved in hypothalamic regulation of feeding and body mass. These can be sorted based on their behavioral effects into anabolic/orexigenic peptides that promote feeding and increase of body mass or as catabolic/anorexigenic peptides. Each set of peptides includes what appears to be a key signal molecule that promotes either feeding or satiety as well as an antagonist of the opposing stimulus.
 |
|
Figure 4.5 |
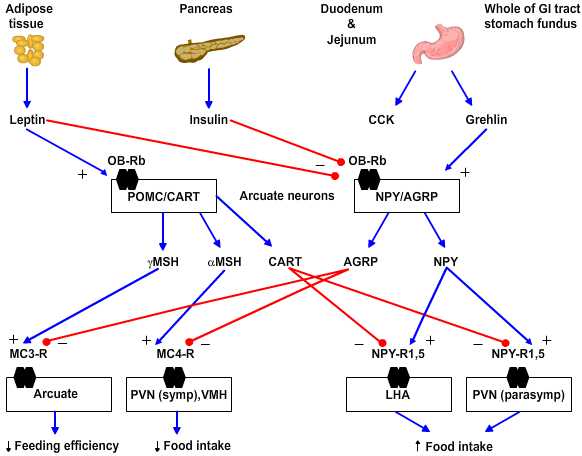
- Anabolic/orexigenic peptides include neuropeptide Y (NPY), agouti-related protein (AGRP), melanin-concentrating hormone (MCH), orexin, and galanin. NPY is the key feeding-promoting neuropeptide and NPY receptor subtype 5 is the key site of action. NPY increases in the arcuate nucleus within 6 hours of food deprivation. Of note the neurons that express NPY in rodents also express receptors for leptin and insulin and so are yoked to plasma-derived feeding signals. AGRP is an antagonist for the anorexigenic peptides as it blocks the MC3 and 4 receptors for γ- and α-melanocyte-stimulating hormones, respectively.
- Catabolic/anorexigenic peptides include a-melanocyte stimulating hormone (a-MSH), cocaine- and amphetamine-regulated transcript (CART), glucagon-like peptides 1 and 2 (GLP-1, GLP-2) and prolactin-releasing peptide (PrlRP). These peptides fall with food deprivation and conversely rise with forced overfeeding. For example, corticotropin-releasing factor (CRF) increases in the paraventricular nucleus (PVN) and pro-opiomelanocortin (POMC) increases in the arcuate nucleus with overfeeding. The key anorexigenic signals are α- and less so γ-MSH acting at the MC4 and MC3 receptors, respectively. The antagonist in this group for the orexigenic peptide NPY is CART.
4.4 Disorders of Feeding and Satiety
Transition sentence/paragraph needed here
Obesity is characterized by an excess of adipose tissue, however, the exact definition of “excess” is somewhat enigmatic. The simplest way to define “excess” is that amount of adipose tissue that creates a health risk. The best index at present is that this represents an amount of adipose tissue that puts an individual 20% or greater above their ideal body weight. Using these criteria somewhere between 20 and 40 percent of adults are obese with these rates over-representative by minorities and the poor. Excess intake of calories is the usual major contributor to obesity. Basal metabolic rate in a 70kg man is about 1500 calories/day, so anything above this in a sedentary, healthy individual will result in weight gain. Another mechanism besides excess caloric intake that may contribute to obesity is the lipoprotein lipase hypothesis in which an excess of adipose tissue lipoprotein lipase preferentially stores lipid calories as adipose tissue. Obesity has several important metabolic sequela that can contribute to additional medical problems.
- Hyperinsulinemia with hyper- or euglycemia. Insulin resistance may develop due to an abnormal beta cell product, circulating antibodies or tissue insulin insensitivity. Insulin insensitivity results due to a combination of receptor and post-receptor defects in insulin action.
- Hyperlipoproteinemia. Obesity is characterized by elevated VLDL and elevated serum triglyceride and an increase in serum free fatty acid turnover.
- Hypertension, Hypoventilation (Pickwickian Syndrome) are often observed in the obese patient. Other endocrine disorders may also develop.
Froehlich’s Syndrome is characterized by obesity, hypogonadotropic hypogonadism and other variable features including diabetes insipidus, visual impairment and mental retardation. It is thought to result due to a hypothalamic lesion.
Anorexia Nervosa is a disorder almost exclusively seen in young, white women of middle-class background, with a prevalence of as high as 1 per 100 being reported. Subclinical prevalence may be as high as 5 percent. Diagnosis is made on a clinical basis as there is no diagnostic test. Patients observed to be below about 80% of ideal body weight are suspect when no other medical (psychological) causes can be identified. A disordered attitude toward eating, food or weight that overrides hunger, ritualized exercise, amenorrhea, bradycardia, hypotension, and hypothermia complete the usual clinical picture. Patients with anorexia are vulnerable to sudden death from ventricular tachyarrhythmias. There is no specific treatment as yet because the underlying mechanisms are not defined. However, a recent study has shown that a significant number of these patients and those with bulimia have plasma antibodies against pituitary and hypothalamic melanotropes and/or corticotropes suggesting an underlying autoimmune disorder.
Bulimia is often considered a disorder related to anorexia nervosa. The disorder is characterized by the episodic ingestion of large amounts of food in a compulsive fashion (“ox-hunger”) coupled with the awareness that the eating pattern is abnormal, that it cannot be stopped, and depression at completion of the act. There is usually a morbid fear of becoming fat, although body weight is usually in the normal range. Induced vomiting that eventually becomes reflexive usually follows episodes of binge eating. Patients frequently have additional behavioral/psychiatric abnormalities.
4.5 Summary
Caloric homeostasis is maintained by a body weight set point. This set point is at least in part maintained by a set of signals from peripheral energy stores into the brain that stimulate feeding to maintain adequate stores of nutrients. Feeding also appears to largely occur as a primed response whenever food is available and that continues until inhibitory satiety signals are received in the brain that are derived from a meal.
Cessation of feeding in a given meal and the time to the next meal is determined by neural and humoral signals derived from that meal. The neural signals come from the vagus and include information concerning the size (stretch of stomach) and caloric/nutrient content (portal glucose, plasma insulin, leptin).
The CNS satiety network is a subdivision of the central autonomic network. Thus, it includes the brainstem afferent and efferent relays for the vagus as well as the brainstem sympathetic relays such as nuclear group A2 and the raphe system. The arcuate nucleus and less so the area postrema are key sites for registration of humoral inputs to this network. Integrated activity between the ventromedial, dorsomedial and lateral hypothalamic areas ultimately is integrated at the paraventricular nucleus that determines a net balance between the feeding promoting central parasympathetic circuitry and the satiety-promoting central sympathetic circuitry.
Chapter 4 http://nba.uth.tmc.edu/neuroscience/s4/chapter04.html
This blog post is shared here as an educational resource for my clients, reference tool, and shared for posterity, to preserve information for future use and promote learning and debate.